A review of ferric citrate clinical studies, and the rationale and design of the Ferric Citrate and Chronic Kidney Disease in Children (FIT4KiD) trial
- PMID: 35237863
- PMCID: PMC9437144
- DOI: 10.1007/s00467-022-05492-7
A review of ferric citrate clinical studies, and the rationale and design of the Ferric Citrate and Chronic Kidney Disease in Children (FIT4KiD) trial
Abstract
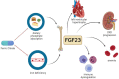
Pediatric chronic kidney disease (CKD) is characterized by many co-morbidities, including impaired growth and development, CKD-mineral and bone disorder, anemia, dysregulated iron metabolism, and cardiovascular disease. In pediatric CKD cohorts, higher circulating concentrations of fibroblast growth factor 23 (FGF23) are associated with some of these adverse clinical outcomes, including CKD progression and left ventricular hypertrophy. It is hypothesized that lowering FGF23 levels will reduce the risk of these events and improve clinical outcomes. Reducing FGF23 levels in CKD may be accomplished by targeting two key stimuli of FGF23 production-dietary phosphate absorption and iron deficiency. Ferric citrate is approved for use as an enteral phosphate binder and iron replacement product in adults with CKD. Clinical trials in adult CKD cohorts have also demonstrated that ferric citrate decreases circulating FGF23 concentrations. This review outlines the possible deleterious effects of excess FGF23 in CKD, summarizes data from the adult CKD clinical trials of ferric citrate, and presents the Ferric Citrate and Chronic Kidney Disease in Children (FIT4KiD) study, a randomized, placebo-controlled trial to evaluate the effects of ferric citrate on FGF23 in pediatric patients with CKD stages 3-4 (ClinicalTrials.gov Identifier NCT04741646).
Keywords: Chronic kidney disease; Ferric citrate; Fibroblast growth factor 23; Pediatrics.
© 2022. The Author(s).
Conflict of interest statement
MRH has received research funding from Akebia Therapeutics, Inc. BAW and IBS are consultants for Akebia Therapeutics, Inc.
Figures