Safety and Pharmacokinetics of Intranasally Administered Heparin
- PMID: 35237922
- PMCID: PMC8890767
- DOI: 10.1007/s11095-022-03191-4
Safety and Pharmacokinetics of Intranasally Administered Heparin
Abstract
Purpose: Intranasally administered unfractionated heparin (UFH) and other sulfated polysaccharides are potential prophylactics for COVID-19. The purpose of this research was to measure the safety and pharmacokinetics of clearance of intranasally administered UFH solution from the nasal cavity.
Methods: Double-blinded daily intranasal dosing in C57Bl6 mice with four doses (60 ng to 60 μg) of UFH was carried out for fourteen consecutive days, with both blood coagulation measurements and subject adverse event monitoring. The pharmacokinetics of fluorescent-labeled UFH clearance from the nasal cavity were measured in mice by in vivo imaging. Intranasal UFH at 2000 U/day solution with nasal spray device was tested for safety in a small number of healthy human subjects.
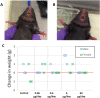
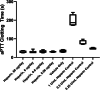
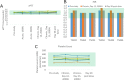
Results: UFH showed no evidence of toxicity in mice at any dose measured. No significant changes were observed in activated partial thromboplastin time (aPTT), platelet count, or frequency of minor irritant events over vehicle-only control. Human subjects showed no significant changes in aPTT time, international normalized ratio (INR), or platelet count over baseline measurements. No serious adverse events were observed. In vivo imaging in a mouse model showed a single phase clearance of UFH from the nasal cavity. After 12 h, 3.2% of the administered UFH remained in the nasal cavity, decaying to background levels by 48 h.
Conclusions: UFH showed no toxic effects for extended daily intranasal dosing in mice as well as humans. The clearance kinetics of intranasal heparin solution from the nasal cavity indicates potentially protective levels for up to 12 h after dosing.
Keywords: Covid-19; SARS-CoV-2; heparin; intranasal delivery.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Figures




Update of
-
Safety and Pharmacokinetics of Intranasally Administered Heparin.medRxiv [Preprint]. 2022 Feb 17:2021.07.05.21259936. doi: 10.1101/2021.07.05.21259936. medRxiv. 2022. Update in: Pharm Res. 2022 Mar;39(3):541-551. doi: 10.1007/s11095-022-03191-4. PMID: 35194614 Free PMC article. Updated. Preprint.
References
-
- Johns Hopkins Coronavirus Resource Center. Available from: https://coronavirus.jhu.edu/.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

