Intravital Microscopy in Atherosclerosis Research
- PMID: 35237994
- PMCID: PMC9052099
- DOI: 10.1007/978-1-0716-1924-7_40
Intravital Microscopy in Atherosclerosis Research
Abstract
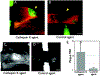
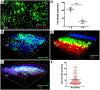
Atherosclerosis is a lipid-driven inflammatory disorder that narrows the arterial lumen and can induce life-threatening complications from coronary artery disease, cerebrovascular disease, and peripheral artery disease. On a mechanistic level, the development of novel cellular-resolution intravital microscopy imaging approaches has recently enabled in vivo studies of underlying biological processes governing disease onset and progress. In particular, multiphoton microscopy has emerged as a promising intravital imaging tool utilizing two-photon-excited fluorescence and second-harmonic generation that provides subcellular resolution and increased imaging depths beyond confocal and epifluorescence microscopy. In this chapter, we describe the state-of-the-art multiphoton microscopy applied to the study of murine atherosclerosis.
Keywords: Atherosclerosis; Gated microscopy; Inflammation; Intravital imaging; Intravital microscopy; Molecular imaging; Multiphoton microscopy.
© 2022. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
Figures

References
-
- Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK (2001) In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med 7:864–868 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

