Controlling ion channel function with renewable recombinant antibodies
- PMID: 35238051
- PMCID: PMC9058206
- DOI: 10.1113/JP282403
Controlling ion channel function with renewable recombinant antibodies
Abstract
Selective ion channel modulators play a critical role in physiology in defining the contribution of specific ion channels to physiological function and as proof of concept for novel therapeutic strategies. Antibodies are valuable research tools that have broad uses including defining the expression and localization of ion channels in native tissue, and capturing ion channel proteins for subsequent analyses. In this review, we detail how renewable and recombinant antibodies can be used to control ion channel function. We describe the different forms of renewable and recombinant antibodies that have been used and the mechanisms by which they modulate ion channel function. We highlight the use of recombinant antibodies that are expressed intracellularly (intrabodies) as genetically encoded tools to control ion channel function. We also offer perspectives of avenues of future research that may be opened by the application of emerging technologies for engineering recombinant antibodies for enhanced utility in ion channel research. Overall, this review provides insights that may help stimulate and guide interested researchers to develop and incorporate renewable and recombinant antibodies as valuable tools to control ion channel function.
Keywords: antibody; intrabody; ion channel modulation; nanobody; scFv.
© 2022 The Authors. The Journal of Physiology © 2022 The Physiological Society.
Conflict of interest statement
Additional information section
Competing Interests: H.M.C is co-founder of Stablix, Inc. J. S. T. declares no competing interests.
Figures
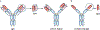
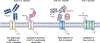
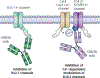

Similar articles
-
Genetically encoded intrabodies as high-precision tools to visualize and manipulate neuronal function.Semin Cell Dev Biol. 2022 Jun;126:117-124. doi: 10.1016/j.semcdb.2021.11.004. Epub 2021 Nov 12. Semin Cell Dev Biol. 2022. PMID: 34782184 Free PMC article.
-
Intracellular antibodies (intrabodies) and their therapeutic potential.Handb Exp Pharmacol. 2008;(181):343-73. doi: 10.1007/978-3-540-73259-4_15. Handb Exp Pharmacol. 2008. PMID: 18071953 Review.
-
Ion channel engineering: perspectives and strategies.J Mol Biol. 2015 Jan 16;427(1):190-204. doi: 10.1016/j.jmb.2014.09.001. Epub 2014 Sep 7. J Mol Biol. 2015. PMID: 25205552 Free PMC article. Review.
-
Making cell-permeable antibodies (Transbody) through fusion of protein transduction domains (PTD) with single chain variable fragment (scFv) antibodies: potential advantages over antibodies expressed within the intracellular environment (Intrabody).Med Hypotheses. 2005;64(6):1105-8. doi: 10.1016/j.mehy.2005.01.011. Med Hypotheses. 2005. PMID: 15823695
-
Ion channel engineering for modulation and de novo generation of electrical excitability.Curr Opin Biotechnol. 2019 Aug;58:100-107. doi: 10.1016/j.copbio.2019.01.004. Epub 2019 Feb 16. Curr Opin Biotechnol. 2019. PMID: 30776744 Free PMC article. Review.
Cited by
-
Extracellular modulation of TREK-2 activity with nanobodies provides insight into the mechanisms of K2P channel regulation.Nat Commun. 2024 May 16;15(1):4173. doi: 10.1038/s41467-024-48536-2. Nat Commun. 2024. PMID: 38755204 Free PMC article.
-
Long-Term Blockade of Nociceptive Nav1.7 Channels Is Analgesic in Rat Models of Knee Arthritis.Biomolecules. 2022 Oct 26;12(11):1571. doi: 10.3390/biom12111571. Biomolecules. 2022. PMID: 36358921 Free PMC article.
-
Ion channel inhibition by targeted recruitment of NEDD4-2 with divalent nanobodies.bioRxiv [Preprint]. 2024 May 31:2024.05.28.596281. doi: 10.1101/2024.05.28.596281. bioRxiv. 2024. PMID: 38854018 Free PMC article. Preprint.
References
-
- Alexander SP, Mathie A, Peters JA, Veale EL, Striessnig J, Kelly E, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Southan C, Davies JA, Aldrich RW, Attali B, Baggetta AM, Becirovic E, Biel M, Bill RM, Catterall WA, Conner AC, Davies P, Delling M, Virgilio FD, Falzoni S, Fenske S, George C, Goldstein SAN, Grissmer S, Ha K, Hammelmann V, Hanukoglu I, Jarvis M, Jensen AA, Kaczmarek LK, Kellenberger S, Kennedy C, King B, Kitchen P, Lynch JW, Perez-Reyes E, Plant LD, Rash L, Ren D, Salman MM, Sivilotti LG, Smart TG, Snutch TP, Tian J, Trimmer JS, Van den Eynde C, Vriens J, Wei AD, Winn BT, Wulff H, Xu H, Yue L, Zhang X & Zhu M. (2021). The concise guide to pharmacology 2021/22: Ion channels. Br J Pharmacol 178 Suppl 1, S157–S245. - PubMed
-
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS & Rhodes KJ. (2000). Modulation of A-type potassium channels by a family of calcium sensors. Nature 403, 553–556. - PubMed
-
- Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JD, Hameed S, Zamponi GW & Turner RW. (2010). Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci 13, 333–337. - PubMed

