Prevalence of anti-severe acute respiratory syndrome coronavirus 2 antibodies in cats in Germany and other European countries in the early phase of the coronavirus disease-19 pandemic
- PMID: 35238485
- PMCID: PMC9115359
- DOI: 10.1111/zph.12932
Prevalence of anti-severe acute respiratory syndrome coronavirus 2 antibodies in cats in Germany and other European countries in the early phase of the coronavirus disease-19 pandemic
Abstract
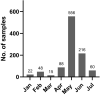
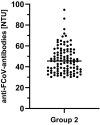
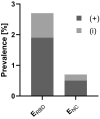
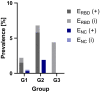
During the first months of the coronavirus disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), cases of human-to-cat transmission were reported. Seroconversion was shown in cats infected under experimental and natural conditions. This large-scale survey of 1,005 serum samples was conducted to investigate anti-SARS-CoV-2 antibody prevalence in domestic cats during the first 7 months of the pandemic in Germany and other European countries. In addition, we compared the sensitivity and specificity of two multispecies SARS-CoV-2 antibody enzyme-linked immunosorbent assays (ELISA). Results were confirmed by using an indirect immunofluorescence test (iIFT) and a surrogate virus neutralization test (sVNT). Sera that were highly positive for feline coronavirus (FCoV) antibodies (n = 103) were included to correct for cross-reactivity of the tests used. Our results showed an overall SARS-CoV-2 seropositivity of 1.9% (n = 19) in a receptor-binding domain (RBD)-based ELISA, additional 0.8% (n = 8) were giving inconclusive results. In contrast, a nucleocapsid-based ELISA revealed 0.5% (n = 5) positive and 0.2% (n = 2) inconclusive results. While the iIFT and sVNT confirmed 100% of positive and 50%-57.1% of the doubtful results as determined in the RBD ELISA, the nucleocapsid-based assay showed a high discrepancy and only one of the five positive results could be confirmed. The results indicate significant deficits of the nucleocapsid-based ELISA with respect to sensitivity and specificity. Due to a significantly higher rate (5.8%) of positive results in the group of highly FCoV antibody-positive samples, cross-reactivity of the FCoV-ELISA with SARS-CoV-2 antibodies cannot be excluded. Furthermore, we investigated the impact of direct contact of domestic cats (n = 23) to SARS-CoV-2 positive owners. Considering one inconclusive result, which got confirmed by iIFT, this exposure did not lead to a significantly higher prevalence (4.4%; p = .358) among tested subjects. Overall, we conclude that cats are a negligible entity with respect to virus transmission in Europe.
Keywords: COVID-19; SARS-CoV-2; cats; seroprevalence.
© 2022 The Authors. Zoonoses and Public Health published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
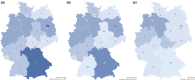
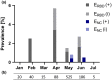
References
-
- Addie, D. , Belák, S. , Boucraut‐Baralon, C. , Egberink, H. , Frymus, T. , Gruffydd‐Jones, T. , Hartmann, K. , Hosie, M. J. , Lloret, A. , Lutz, H. , Marsilio, F. , Pennisi, M. G. , Radford, A. D. , Thiry, E. , Truyen, U. , & Horzinek, M. C. (2009). Feline infectious peritonitis. ABCD guidelines on prevention and management. Journal of Feline Medicine & Surgery, 11(7), 594–604. 10.1016/j.jfms.2009.05.008 - DOI - PMC - PubMed
-
- Amanat, F. , Stadlbauer, D. , Strohmeier, S. , Nguyen, T. H. O. , Chromikova, V. , McMahon, M. , Jiang, K. , Arunkumar, G. A. , Jurczyszak, D. , Polanco, J. , Bermudez‐Gonzalez, M. , Kleiner, G. , Aydillo, T. , Miorin, L. , Fierer, D. S. , Lugo, L. A. , Kojic, E. M. , Stoever, J. , Liu, S. T. H. , … Krammer, F. (2020). A serological assay to detect SARS‐CoV‐2 seroconversion in humans. Nature Medicine, 26(7), 1033–1036. 10.1038/s41591-020-0913-5 - DOI - PMC - PubMed
-
- Bosco‐Lauth, A. M. , Hartwig, A. E. , Porter, S. M. , Gordy, P. W. , Nehring, M. , Byas, A. D. , VandeWoude, S. , Ragan, I. K. , Maison, R. M. , & Bowen, R. A. (2020). Experimental infection of domestic dogs and cats with SARS‐CoV‐2: Pathogenesis, transmission, and response to reexposure in cats. Proceedings of the National Academy of Sciences of the United States of America, 117(42), 26382–26388. 10.1073/pnas.2013102117 - DOI - PMC - PubMed
-
- Chia, W. N. , Tan, C. W. , Foo, R. , Kang, A. E. Z. , Peng, Y. , Sivalingam, V. , Tiu, C. , Ong, X. M. , Zhu, F. , Young, B. E. , Chen, M.‐C. , Tan, Y.‐J. , Lye, D. C. , Anderson, D. E. , & Wang, L.‐F. (2020). Serological differentiation between COVID‐19 and SARS infections. Emerging Microbes & Infections, 9(1), 1497–1505. 10.1080/22221751.2020.1780951 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

