Targeting ATP-Sensitive K+ Channels to Treat Pulmonary Hypertension
- PMID: 35238728
- PMCID: PMC9116352
- DOI: 10.1165/rcmb.2021-0549ED
Targeting ATP-Sensitive K+ Channels to Treat Pulmonary Hypertension
Figures
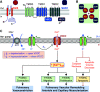
Comment on
-
SUR1 As a New Therapeutic Target for Pulmonary Arterial Hypertension.Am J Respir Cell Mol Biol. 2022 May;66(5):539-554. doi: 10.1165/rcmb.2021-0180OC. Am J Respir Cell Mol Biol. 2022. PMID: 35175177
References
-
- Montani D, Chaumais MC, Guignabert C, Günther S, Girerd B, Jaïs X, et al. Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther . 2014;141:172–191. - PubMed
-
- Le Ribeuz H, Masson B, Capuano V, Dutheil M, Gooroochurn H, Boët A, et al. SUR1 as a new therapeutic target for pulmonary arterial hypertension. Am J Respir Cell Mol Biol . 2022;66 - PubMed
-
- Papp R, Nagaraj C, Zabini D, Nagy BM, Lengyel M, Skofic Maurer D, et al. Targeting TMEM16A to reverse vasoconstriction and remodelling in idiopathic pulmonary arterial hypertension. Eur Respir J . 2019;53:1800965. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

