A human-derived 3D brain organoid model to study JC virus infection
- PMID: 35239145
- PMCID: PMC8892818
- DOI: 10.1007/s13365-022-01062-7
A human-derived 3D brain organoid model to study JC virus infection
Abstract
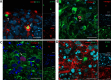
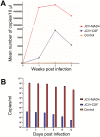
Progressive multifocal leukoencephalopathy (PML) is a frequent neurological complication in immunosuppressed patients. PML is caused by the JC virus (JCV), a neurotropic DNA polyomavirus that infects oligodendrocytes and astrocytes, causing inflammation and demyelination which lead to neurological dysfunction. The pathogenesis of PML is poorly understood due to the lack of in vitro or animal models to study mechanisms of disease as the virus most efficiently infects only human cells. We developed a human-derived brain organotypic system (also called brain organoid) to model JCV infection. The model was developed by using human-induced pluripotent stem cells (iPSC) and culturing them in 3D to generate an organotypic model containing neurons, astrocytes, and oligodendrocytes which recapitulates aspects of the environment of the human brain. We infected the brain organoids with the JCV MAD4 strain or cerebrospinal fluid of a patient with PML. The organoids were assessed for evidence of infection by qPCR, immunofluorescence, and electron microscopy at 1, 2, and 3 weeks post-exposure. JCV infection in both JCV MAD4 strain and PML CSF-exposed brain organoids was confirmed by immunocytochemical studies demonstrating viral antigens and electron microscopy showing virion particles in the nuclear compartment of oligodendrocytes and astrocytes. No evidence of neuronal infection was visualized. Infection was also demonstrated by JCV qPCR in the virus-exposed organoids and their media. In conclusion, the brain organoid model of JCV infection establishes a human model suitable for studying the mechanisms of JCV infection and pathogenesis of PML and may facilitate the exploration of therapeutic approaches.
Keywords: Human cells; In vitro model; JC virus; JCV; Microphysiological system; Organoid.
© 2022. Journal of NeuroVirology, Inc.
Conflict of interest statement
TH, HH, and DP are named inventors on a patent by Johns Hopkins University on the production of mini-brains (also called BrainSpheres), which is licensed to AxoSim, New Orleans, LA, USA. They consult AxoSim and TH and HTH are shareholders. The rest of the authors have no disclosures.
Figures


Similar articles
-
Frequency and large T (LT) sequence of JC polyomavirus DNA in oligodendrocytes, astrocytes and granular cells in non-PML brain.Brain Pathol. 2012 May;22(3):329-36. doi: 10.1111/j.1750-3639.2011.00538.x. Epub 2011 Oct 27. Brain Pathol. 2012. PMID: 21951346 Free PMC article.
-
JC virus infection of meningeal and choroid plexus cells in patients with progressive multifocal leukoencephalopathy.J Neurovirol. 2019 Aug;25(4):520-524. doi: 10.1007/s13365-019-00753-y. Epub 2019 Apr 25. J Neurovirol. 2019. PMID: 31025264 Free PMC article.
-
Progressive multifocal leukoencephalopathy in Zambia is caused by JC virus with prototype regulatory region.J Neurovirol. 2019 Aug;25(4):475-479. doi: 10.1007/s13365-019-00746-x. Epub 2019 Apr 26. J Neurovirol. 2019. PMID: 31028690 Free PMC article.
-
Revisiting JC virus and progressive multifocal leukoencephalopathy.J Neurovirol. 2023 Oct;29(5):524-537. doi: 10.1007/s13365-023-01164-w. Epub 2023 Sep 2. J Neurovirol. 2023. PMID: 37659983 Review.
-
The Role of the JC Virus in Central Nervous System Tumorigenesis.Int J Mol Sci. 2020 Aug 28;21(17):6236. doi: 10.3390/ijms21176236. Int J Mol Sci. 2020. PMID: 32872288 Free PMC article. Review.
Cited by
-
The Inhibition of DNA Viruses by the Amphibian Antimicrobial Peptide Temporin G: A Virological Study Addressing HSV-1 and JPCyV.Int J Mol Sci. 2022 Jun 28;23(13):7194. doi: 10.3390/ijms23137194. Int J Mol Sci. 2022. PMID: 35806198 Free PMC article.
-
Report of the Assay Guidance Workshop on 3-Dimensional Tissue Models for Antiviral Drug Development.J Infect Dis. 2023 Oct 3;228(Suppl 5):S337-S354. doi: 10.1093/infdis/jiad334. J Infect Dis. 2023. PMID: 37669225 Free PMC article.
-
Human Brain Organoids as Models for Central Nervous System Viral Infection.Viruses. 2022 Mar 18;14(3):634. doi: 10.3390/v14030634. Viruses. 2022. PMID: 35337041 Free PMC article. Review.
-
CRISPR antiviral inhibits neurotrophic JC polyomavirus in 2D and 3D culture models through dual-gRNA excision by SaCas9.Mol Ther Nucleic Acids. 2025 May 14;36(2):102556. doi: 10.1016/j.omtn.2025.102556. eCollection 2025 Jun 10. Mol Ther Nucleic Acids. 2025. PMID: 40510594 Free PMC article.
-
Critical analysis of translational potential of rodent models of white matter pathology across a wide spectrum of human diseases.Cell Death Dis. 2025 Jul 31;16(1):580. doi: 10.1038/s41419-025-07893-6. Cell Death Dis. 2025. PMID: 40744926 Free PMC article. Review.
References
-
- Adang L, Berger J (2015) Progressive multifocal leukoencephalopathy. F1000Research, 4(F1000 Faculty Rev-1424.) 10.12688/f1000research.7071.1 - PubMed
-
- Bullen CK, Hogberg HT, Bahadirli-Talbott A, Bishai WR, Hartung T, Keuthan C, Looney MM, Pekosz A, Romero JC, Sillé FCM, Um P, Smirnova L (2020) Infectability of human BrainSphere neurons suggests neurotropism of SARS-CoV-2. Altex 37(4):665–671. 10.14573/altex.2006111 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

