Boosting the Discovery of Small Molecule Inhibitors of Glucose-6-Phosphate Dehydrogenase for the Treatment of Cancer, Infectious Diseases, and Inflammation
- PMID: 35239352
- PMCID: PMC9553131
- DOI: 10.1021/acs.jmedchem.1c01577
Boosting the Discovery of Small Molecule Inhibitors of Glucose-6-Phosphate Dehydrogenase for the Treatment of Cancer, Infectious Diseases, and Inflammation
Abstract
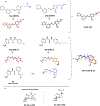
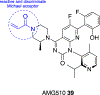
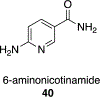
We present an overview of small molecule glucose-6-phosphate dehydrogenase (G6PD) inhibitors that have potential for use in the treatment of cancer, infectious diseases, and inflammation. Both steroidal and nonsteroidal inhibitors have been identified with steroidal inhibitors lacking target selectivity. The main scaffolds encountered in nonsteroidal inhibitors are quinazolinones and benzothiazinones/benzothiazepinones. Three molecules show promise for development as antiparasitic (25 and 29) and anti-inflammatory (32) agents. Regarding modality of inhibition (MOI), steroidal inhibitors have been shown to be uncompetitive and reversible. Nonsteroidal small molecules have exhibited all types of MOI. Strategies to boost the discovery of small molecule G6PD inhibitors include exploration of structure-activity relationships (SARs) for established inhibitors, employment of high-throughput screening (HTS), and fragment-based drug discovery (FBDD) for the identification of new hits. We discuss the challenges and gaps associated with drug discovery efforts of G6PD inhibitors from in silico, in vitro, and in cellulo to in vivo studies.
Conflict of interest statement
We declare no competing interests.
Figures


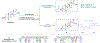
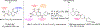



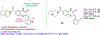
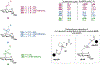
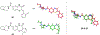


References
-
- Tian W-N; Braunstein LD; Pang J; Stuhlmeier KM; Xi Q-C; Tian X; Stanton RC, Importance of Glucose-6-phosphate Dehydrogenase Activity for Cell Growth. J. Biol. Chem 1998, 273 (17), 10609–10617. - PubMed
-
- Hutchings D; Rawsthorne S; Emes MJ, Fatty Acid Synthesis and the Oxidative Pentose Phosphate Pathway in Developing Embryos of Oilseed Rape (Brassica napus L.). J. Exp. Bot 2004, 56 (412), 577–585. - PubMed
-
- Kornberg A; Horecker BL; Horecker BL; Smyrniotis PZ, [42] Glucose-6-phosphate dehydrogenase 6-Phosphogluconic Dehydrogenase. In Methods in Enzymology, Academic Press: 1955; Vol. 1, pp 323–327.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Miscellaneous

