Worldwide Control and Management of Chagas Disease in a New Era of Globalization: a Close Look at Congenital Trypanosoma cruzi Infection
- PMID: 35239422
- PMCID: PMC9020358
- DOI: 10.1128/cmr.00152-21
Worldwide Control and Management of Chagas Disease in a New Era of Globalization: a Close Look at Congenital Trypanosoma cruzi Infection
Abstract
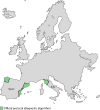
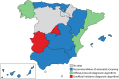
Population movements have turned Chagas disease (CD) into a global public health problem. Despite the successful implementation of subregional initiatives to control vectorial and transfusional Trypanosoma cruzi transmission in Latin American settings where the disease is endemic, congenital CD (cCD) remains a significant challenge. In countries where the disease is not endemic, vertical transmission plays a key role in CD expansion and is the main focus of its control. Although several health organizations provide general protocols for cCD control, its management in each geopolitical region depends on local authorities, which has resulted in a multitude of approaches. The aims of this review are to (i) describe the current global situation in CD management, with emphasis on congenital infection, and (ii) summarize the spectrum of available strategies, both official and unofficial, for cCD prevention and control in countries of endemicity and nonendemicity. From an economic point of view, the early detection and treatment of cCD are cost-effective. However, in countries where the disease is not endemic, national health policies for cCD control are nonexistent, and official regional protocols are scarce and restricted to Europe. Countries of endemicity have more protocols in place, but the implementation of diagnostic methods is hampered by economic constraints. Moreover, most protocols in both countries where the disease is endemic and those where it is not endemic have yet to incorporate recently developed technologies. The wide methodological diversity in cCD diagnostic algorithms reflects the lack of a consensus. This review may represent a first step toward the development of a common strategy, which will require the collaboration of health organizations, governments, and experts in the field.
Keywords: Trypanosoma cruzi; congenital Chagas disease; diagnosis; endemic; health policies; nonendemic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Chagas C. 1909. Nova tripanozomiaze humana. Estudos sobre a morfolojia e o ciclo evolutivo de Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz 1:159–218. 10.1590/S0074-02761909000200008. - DOI
-
- Pan American Health Organization. 2017. EMTCT Plus: framework for elimination of mother-to-child transmission of HIV, syphilis, hepatitis B, and Chagas. OPS/CHA/17-009. Pan American Health Organization, Washington, DC. https://iris.paho.org/handle/10665.2/34306. Accessed 21 December 2021.
-
- Carlier Y, Truyens C. 2017. Maternal-fetal transmission of Trypanosoma cruzi, p 517–559. In Telleria J, Tibayrenc M (ed), American trypanosomiasis—Chagas diseases. One hundred years of research, 1st ed. Elsevier, Burlington, MA. 10.1016/B978-0-12-801029-7.00024-1. - DOI
-
- Carlier Y, Altcheh J, Angheben A, Freilij H, Luquetti AO, Schijman AG, Segovia M, Wagner N, Albajar Viñas P. 2019. Congenital Chagas disease: updated recommendations for prevention, diagnosis, treatment, and follow-up of newborns and siblings, girls, women of childbearing age, and pregnant women. PLoS Negl Trop Dis 13:e0007694. 10.1371/journal.pntd.0007694. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

