Dual-function Spot 42 RNA encodes a 15-amino acid protein that regulates the CRP transcription factor
- PMID: 35239441
- PMCID: PMC8916003
- DOI: 10.1073/pnas.2119866119
Dual-function Spot 42 RNA encodes a 15-amino acid protein that regulates the CRP transcription factor
Abstract
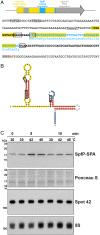
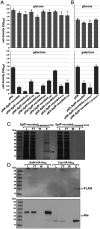
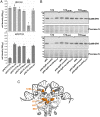
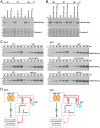
SignificanceDual-function RNAs base pair with target messenger RNAs as small regulatory RNAs and encode small protein regulators. However, only a limited number of these dual-function regulators have been identified. In this study, we show that a well-characterized base-pairing small RNA surprisingly also encodes a 15-amino acid protein. The very small protein binds the cyclic adenosine monophosphate receptor protein transcription factor to block activation of some promoters, raising the question of how many other transcription factors are modulated by unidentified small proteins.
Keywords: SpfP; carbon catabolite repression; dual-function RNA; sRNA; small protein.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Brückner R., Titgemeyer F., Carbon catabolite repression in bacteria: Choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 209, 141–148 (2002). - PubMed
-
- Görke B., Stülke J., Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6, 613–624 (2008). - PubMed
-
- Kolb A., Busby S., Buc H., Garges S., Adhya S., Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62, 749–795 (1993). - PubMed
-
- Busby S., Ebright R. H., Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293, 199–213 (1999). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

