Study protocol for the Innovative Support for Patients with SARS-COV-2 Infections Registry (INSPIRE): A longitudinal study of the medium and long-term sequelae of SARS-CoV-2 infection
- PMID: 35239680
- PMCID: PMC8893622
- DOI: 10.1371/journal.pone.0264260
Study protocol for the Innovative Support for Patients with SARS-COV-2 Infections Registry (INSPIRE): A longitudinal study of the medium and long-term sequelae of SARS-CoV-2 infection
Abstract
Background: Reports on medium and long-term sequelae of SARS-CoV-2 infections largely lack quantification of incidence and relative risk. We describe the rationale and methods of the Innovative Support for Patients with SARS-CoV-2 Registry (INSPIRE) that combines patient-reported outcomes with data from digital health records to understand predictors and impacts of SARS-CoV-2 infection.
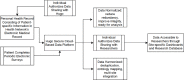
Methods: INSPIRE is a prospective, multicenter, longitudinal study of individuals with symptoms of SARS-CoV-2 infection in eight regions across the US. Adults are eligible for enrollment if they are fluent in English or Spanish, reported symptoms suggestive of acute SARS-CoV-2 infection, and if they are within 42 days of having a SARS-CoV-2 viral test (i.e., nucleic acid amplification test or antigen test), regardless of test results. Recruitment occurs in-person, by phone or email, and through online advertisement. A secure online platform is used to facilitate the collation of consent-related materials, digital health records, and responses to self-administered surveys. Participants are followed for up to 18 months, with patient-reported outcomes collected every three months via survey and linked to concurrent digital health data; follow-up includes no in-person involvement. Our planned enrollment is 4,800 participants, including 2,400 SARS-CoV-2 positive and 2,400 SARS-CoV-2 negative participants (as a concurrent comparison group). These data will allow assessment of longitudinal outcomes from SARS-CoV-2 infection and comparison of the relative risk of outcomes in individuals with and without infection. Patient-reported outcomes include self-reported health function and status, as well as clinical outcomes including health system encounters and new diagnoses.
Results: Participating sites obtained institutional review board approval. Enrollment and follow-up are ongoing.
Conclusions: This study will characterize medium and long-term sequelae of SARS-CoV-2 infection among a diverse population, predictors of sequelae, and their relative risk compared to persons with similar symptomatology but without SARS-CoV-2 infection. These data may inform clinical interventions for individuals with sequelae of SARS-CoV-2 infection.
Conflict of interest statement
I read the journal’s policy and the authors of this manuscript have the following competing interests: HMK is co-founder for Hugo Health; he is not employed by Hugo Health and he receives no salary support from Hugo Health. Hugo Health is a vendor to support INSPIRE study operations and is not the funder of this study or the subject of the investigation. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- CDC. COVID Data Tracker. 28 Mar 2020 [accessed 21 December 2021]. Available: https://covid.cdc.gov/covid-data-tracker/
-
- COVID-19 map—johns Hopkins Coronavirus resource Center. [accessed 21 December 2021]. Available: https://coronavirus.jhu.edu/map.html
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous