Co-transmission of neuropeptides and monoamines choreograph the C. elegans escape response
- PMID: 35239681
- PMCID: PMC8932558
- DOI: 10.1371/journal.pgen.1010091
Co-transmission of neuropeptides and monoamines choreograph the C. elegans escape response
Abstract
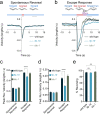
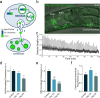
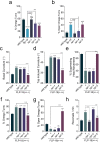
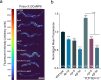
Co-localization and co-transmission of neurotransmitters and neuropeptides is a core property of neural signaling across species. While co-transmission can increase the flexibility of cellular communication, understanding the functional impact on neural dynamics and behavior remains a major challenge. Here we examine the role of neuropeptide/monoamine co-transmission in the orchestration of the C. elegans escape response. The tyraminergic RIM neurons, which coordinate distinct motor programs of the escape response, also co-express the neuropeptide encoding gene flp-18. We find that in response to a mechanical stimulus, flp-18 mutants have defects in locomotory arousal and head bending that facilitate the omega turn. We show that the induction of the escape response leads to the release of FLP-18 neuropeptides. FLP-18 modulates the escape response through the activation of the G-protein coupled receptor NPR-5. FLP-18 increases intracellular calcium levels in neck and body wall muscles to promote body bending. Our results show that FLP-18 and tyramine act in different tissues in both a complementary and antagonistic manner to control distinct motor programs during different phases of the C. elegans flight response. Our study reveals basic principles by which co-transmission of monoamines and neuropeptides orchestrate in arousal and behavior in response to stress.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage; An Account of Recent Researches into the Function of Emotional Excitement. NEW YORK AND LONDON D. APPLETON AND COMPANY; 1915.
-
- Horn JP, Swanson LW. Principles of Neural Science. 5th ed. Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, editors. New York: McGraw Hill; 2013. doi: 10.1126/science.1239052 - DOI
-
- Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: A comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol. 1998;393(2):244–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

