Efficacy and Safety of Maintenance Ustekinumab for Ulcerative Colitis Through 3 Years: UNIFI Long-term Extension
- PMID: 35239968
- PMCID: PMC9426670
- DOI: 10.1093/ecco-jcc/jjac030
Efficacy and Safety of Maintenance Ustekinumab for Ulcerative Colitis Through 3 Years: UNIFI Long-term Extension
Abstract
Background and aims: The UNIFI long-term extension [LTE] study reports the efficacy and safety of subcutaneous 90 mg ustekinumab through 3 years of maintenance therapy.
Methods: Patients randomised to ustekinumab every 12 weeks [q12w] or every 8 weeks [q8w] at maintenance baseline [N = 348] and randomised ustekinumab-treated patients in the LTE [N = 284] were evaluated. Symptomatic remission [Mayo stool frequency = 0/1, rectal bleeding = 0] was assessed. Safety included all LTE patients [N = 188 placebo and N = 457 ustekinumab].
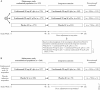
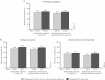
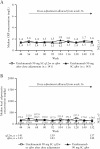
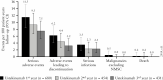
Results: Among patients randomised to the ustekinumab q12w and q8w groups at maintenance baseline, 54.1% and 56.3% achieved symptomatic remission at Week 152, respectively. Overall, 20% of patients discontinued ustekinumab, 10% of biologic-naïve and 30% of biologic-exposed patients. Among patients in symptomatic remission at Year 3, 94.6% and 98.0% of patients were also corticosteroid free, respectively. Corticosteroid-free symptomatic remission rates in the ustekinumab q12w and q8w groups were 51.2% and 55.1% at Week 152, respectively. Remission rates were higher for biologic-naïve patients than for those with a history of biologic failure. Biochemical evidence of response was demonstrated by stable, decreased C-reactive protein and faecal calprotectin measurements over 3 years. From Weeks 96 to 156, no deaths, major adverse cardiovascular events, or tuberculosis occurred. Nasopharyngitis, ulcerative colitis, and upper respiratory tract infection were most frequently reported. One ustekinumab-treated patient with a history of basal cell carcinoma [BCC] reported two BCCs. One patient in the q8w ustekinumab group, who was receiving concomitant 6-mercaptopurine, experienced serious adverse events of neutropenic sepsis and oral herpes.
Conclusions: Efficacy of ustekinumab in patients with ulcerative colitis was confirmed through 3 years. No new safety signals were observed.
Keywords: Ustekinumab; symptomatic remission; ulcerative colitis.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Figures




References
-
- Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365:1713–25. - PubMed
-
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. - PubMed
-
- Sandborn WJ, Feagan BG, Marano C, et al. ; PURSUIT-SC Study Group. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85–95; quiz e14–5. - PubMed
-
- Sandborn WJ, Feagan BG, Marano C, et al. ; PURSUIT-Maintenance Study Group. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:96–109.e1. - PubMed
-
- Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomized controlled trial. Gut 2011;60:780–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

