Cellular therapies for the treatment and prevention of SARS-CoV-2 infection
- PMID: 35240679
- PMCID: PMC8896869
- DOI: 10.1182/blood.2021012249
Cellular therapies for the treatment and prevention of SARS-CoV-2 infection
Abstract
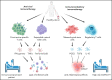
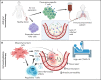
Patients with blood disorders who are immune suppressed are at increased risk for infection with severe acute respiratory syndrome coronavirus 2. Sequelae of infection can include severe respiratory disease and/or prolonged duration of viral shedding. Cellular therapies may protect these vulnerable patients by providing antiviral cellular immunity and/or immune modulation. In this recent review of the field, phase 1/2 trials evaluating adoptive cellular therapies with virus-specific T cells or natural killer cells are described along with trials evaluating the safety, feasibility, and preliminary efficacy of immune modulating cellular therapies including regulatory T cells and mesenchymal stromal cells. In addition, the immunologic basis for these therapies is discussed.
© 2022 by The American Society of Hematology.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

