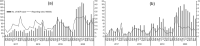Surveillance of adverse events following immunization of 13-valent pneumococcal conjugate vaccine among infants, in Zhejiang province, China
- PMID: 35240930
- PMCID: PMC9009923
- DOI: 10.1080/21645515.2022.2035141
Surveillance of adverse events following immunization of 13-valent pneumococcal conjugate vaccine among infants, in Zhejiang province, China
Abstract
Objectives: To evaluate the safety of 13-valent pneumococcal conjugate vaccine (PCV13) after its licensure.
Methods: Review and describe the AEFI reported to national adverse event following immunization surveillance system (NAEFISS) in Zhejiang province from 2017 to 2020. Reporting rates of AEFI were calculated by age, city, severity of AEFI, categories of AEFI, and reaction categories. The data mining algorithm used in this study was reporting odds ratio (ROR). A value of ROR-1.96SE >1 (standard error [SE]) was considered as the positive signal.
Results: NAEFISS received 3332 AEFI cases following PCV13, with a reporting rate of 17.58/10000 doses. Of the reported AEFI, 652 were serious AEFI cases and the reporting rate was 3.44 for serious AEFI. The reporting rate of fever was the highest among all the clinical diagnosis (7.39/10000 doses). The positive signals were obtained for injection site reaction (ROR-1.96SE: 1.55), hypotonic hyporesponsive episode (HHE) (ROR-1.96SE: 1.62) and febrile seizure (ROR-1.96SE: 1.52).
Conclusion: The present results supported previous observations that the PCV13 administered as the four-dose schedule was generally well tolerated in Chinese infants as we did not identify any new/unexpected safety concern from the NAEFISS during a four-year time period.
Keywords: AEFI (adverse events following immunization); PCV13(13-valent pneumococcal conjugate vaccine); infant; safety; surveillance; vaccination.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. - DOI - PubMed
-
- World Health Organization . Pneumococcal vaccines-WHO position paper 2012. Weekly Epidemiol Record 2012;87:129–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical