Half-life time prediction of developing first-line antiretroviral treatment failure and its risk factors among TB and HIV co-infected children in Northwest Ethiopia; multi setting historical follow-up study
- PMID: 35241036
- PMCID: PMC8892785
- DOI: 10.1186/s12887-022-03177-6
Half-life time prediction of developing first-line antiretroviral treatment failure and its risk factors among TB and HIV co-infected children in Northwest Ethiopia; multi setting historical follow-up study
Abstract
Background: Even though treatment failure is higher among TB and HIV infected children in a resource-limited setting, there is no prior evidence in general and in the study area in particular. Hence, this study was aimed at determining the half-life time prediction of developing first-line antiretroviral treatment failure and its risk factors among TB and HIV co-infected children.
Methods: A historical follow-up study was employed among 239 TB and HIV co-infected children from January 2010-December 2020. The data was entered into Epi data version 4.2.2 and exported to STATA 14.0 Software for analysis. The Kaplan-Meier plot was used to estimate the half-life time to develop treatment failure. The required assumption was fulfilled for each predictor variable. Additionally, those variables having a p-value ≤0.25 in the bivariable analysis were fitted into a multivariable Cox-proportional hazards regression model. P-value, < 0.05 was used to declare a significant association.
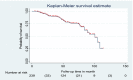
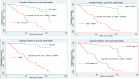
Results: A total of 239 TB and HIV co-infected children were involved in this study. The overall half-life time to develop first treatment failure was found to be 101 months, with a total of 1027.8 years' follow-up period. The incidence rate and proportion of developing first-line treatment failure were 5.5 per 100 PPY (Person-Year) [CI (confidence interval): 3.7, 6.9] 100 PPY and 23.8% (CI; 18.8, 29.7) respectively. Factors such as hemoglobin 10 mg/dl [AHR (Adjusted Hazard Ratio): 3.2 (95% CI: 1.30, 7.73), severe acute malnutrition [AHR: 3.8 (95% CI: 1.51, 79.65), World Health Organization stage IV [AHR: 2.4 (95% CI: 1.15, 4.93)], and cotrimoxazole prophylaxis non user [AHR: 2.3 (95% CI: 1.14, 4.47)] were found to be a risk factor to develop treatment failure.
Conclusion: In this study, the half-life time to develop first-line treatment failure was found to be very low. In addition, the incidence was found to be very high. The presence of hemoglobin 10 mg/dl, severe acute malnutrition, World Health Organization stage, and non-use of cotrimoxazole prophylaxis were discovered to be risk factors for treatment failure. Further prospective cohort and qualitative studies should be conducted to improve the quality of care in paediatric ART clinics to reduce the incidence or burden of first line treatment failure among TB and HIV co-infected children.
Keywords: Children; Ethiopia; Half-life; TB and HIV co-infected; Treatment failure.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
Similar articles
-
Estimation of lifetime survival and predictors of mortality among TB with HIV co-infected children after test and treat strategies launched in Northwest, Ethiopia, 2021; a multicentre historical follow-up study.PLoS One. 2021 Dec 21;16(12):e0258964. doi: 10.1371/journal.pone.0258964. eCollection 2021. PLoS One. 2021. PMID: 34932563 Free PMC article. Clinical Trial.
-
Incidence and Predictors of Major Adverse Drug Reactions Among Human Immunodeficiency Virus-infected Children on Antiretroviral Treatment in West Amhara Comprehensive Specialized Hospitals, Northwest Ethiopia: A Multicenter Retrospective Follow-up Study.Clin Ther. 2024 Feb;46(2):e45-e53. doi: 10.1016/j.clinthera.2023.11.001. Epub 2023 Dec 16. Clin Ther. 2024. PMID: 38105175
-
Incidence and predictors of opportunistic infections among HIV-infected children on antiretroviral therapy at public health facilities of Southwest Ethiopia People Regional State, 2023: a multicenter retrospective follow-up study.BMC Pediatr. 2024 Oct 11;24(1):653. doi: 10.1186/s12887-024-05117-y. BMC Pediatr. 2024. PMID: 39394104 Free PMC article.
-
Onset and predictors of first-line antiretroviral therapy treatment failure among children in Ethiopia: a systematic review and meta-analysis.BMC Pediatr. 2024 Dec 27;24(1):839. doi: 10.1186/s12887-024-05324-7. BMC Pediatr. 2024. PMID: 39731032 Free PMC article.
-
Incidence and predictors of opportunistic infections in adolescents and adults after the initiation of antiretroviral therapy: A 10-year retrospective cohort study in Ethiopia.Front Public Health. 2022 Dec 15;10:1064859. doi: 10.3389/fpubh.2022.1064859. eCollection 2022. Front Public Health. 2022. PMID: 36589962 Free PMC article. Review.
References
-
- Sisay MM, Ayele TA, Gelaw YA, Tsegaye AT, Gelaye KA, Melak MF. Incidence and risk factors of first-line antiretroviral treatment failure among human immunodeficiency virus-infected children in Amhara regional state, Ethiopia: a retrospective follow-up study. BMJ Open. 2018;8(4):e019181. doi: 10.1136/bmjopen-2017-019181. - DOI - PMC - PubMed
-
- Workneh N, Girma T, Woldie M. Immunologic and clinical outcomes of children on HAART: a retrospective cohort analysis at Jimma University specialized hospital. Ethiop J Health Sci. 2009;19(2) Available from: https://www.ajol.info/index.php/ejhs/article/view/69422. [cited 2021 Jan 15].
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

