A Computational Model of Direction Selectivity in Macaque V1 Cortex Based on Dynamic Differences between On and Off Pathways
- PMID: 35241489
- PMCID: PMC9034785
- DOI: 10.1523/JNEUROSCI.2145-21.2022
A Computational Model of Direction Selectivity in Macaque V1 Cortex Based on Dynamic Differences between On and Off Pathways
Abstract
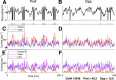
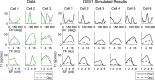
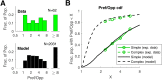
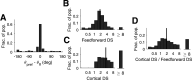
This paper is about neural mechanisms of direction selectivity (DS) in macaque primary visual cortex, V1. We present data (on male macaque) showing strong DS in a majority of simple cells in V1 layer 4Cα, the cortical layer that receives direct afferent input from the magnocellular division of the lateral geniculate nucleus (LGN). Magnocellular LGN cells are not direction-selective. To understand the mechanisms of DS, we built a large-scale, recurrent model of spiking neurons called DSV1. Like its predecessors, DSV1 reproduces many visual response properties of V1 cells including orientation selectivity. Two important new features of DSV1 are (1) DS is initiated by small, consistent dynamic differences in the visual responses of OFF and ON Magnocellular LGN cells, and (2) DS in the responses of most model simple cells is increased over those of their feedforward inputs; this increase is achieved through dynamic interaction of feedforward and intracortical synaptic currents without the use of intracortical direction-specific connections. The DSV1 model emulates experimental data in the following ways: (1) most 4Cα Simple cells were highly direction-selective but 4Cα Complex cells were not; (2) the preferred directions of the model's direction-selective Simple cells were invariant with spatial and temporal frequency (TF); (3) the distribution of the preferred/opposite ratio across the model's population of cells was very close to that found in experiments. The strong quantitative agreement between DS in data and in model simulations suggests that the neural mechanisms of DS in DSV1 may be similar to those in the real visual cortex.SIGNIFICANCE STATEMENT Motion perception is a vital part of our visual experience of the world. In monkeys, whose vision resembles that of humans, the neural computation of the direction of a moving target starts in the primary visual cortex, V1, in layer 4Cα that receives input from the eye through the lateral geniculate nucleus (LGN). How direction selectivity (DS) is generated in layer 4Cα is an outstanding unsolved problem in theoretical neuroscience. In this paper, we offer a solution based on plausible biological mechanisms. We present a new large-scale circuit model in which DS originates from slightly different LGN ON/OFF response time-courses and is enhanced in cortex without the need for direction-specific intracortical connections. The model's DS is in quantitative agreement with experiments.
Keywords: ON/OFF pathways; computational model; direction selectivity; mechanisms; motion perception; primary visual cortex.
Copyright © 2022 the authors.
Figures






Similar articles
-
A theory of direction selectivity for macaque primary visual cortex.Proc Natl Acad Sci U S A. 2021 Aug 10;118(32):e2105062118. doi: 10.1073/pnas.2105062118. Proc Natl Acad Sci U S A. 2021. PMID: 34353906 Free PMC article.
-
Orientation Selectivity from Very Sparse LGN Inputs in a Comprehensive Model of Macaque V1 Cortex.J Neurosci. 2016 Dec 7;36(49):12368-12384. doi: 10.1523/JNEUROSCI.2603-16.2016. J Neurosci. 2016. PMID: 27927956 Free PMC article.
-
Response Selectivity of the Lateral Posterior Nucleus Axons Projecting to the Mouse Primary Visual Cortex.Front Neural Circuits. 2022 Feb 28;16:825735. doi: 10.3389/fncir.2022.825735. eCollection 2022. Front Neural Circuits. 2022. PMID: 35296036 Free PMC article.
-
Mapping the primate lateral geniculate nucleus: a review of experiments and methods.J Physiol Paris. 2014 Feb;108(1):3-10. doi: 10.1016/j.jphysparis.2013.10.001. Epub 2013 Nov 21. J Physiol Paris. 2014. PMID: 24270042 Free PMC article. Review.
-
Functional cell classes and functional architecture in the early visual system of a highly visual rodent.Prog Brain Res. 2005;149:127-45. doi: 10.1016/S0079-6123(05)49010-X. Prog Brain Res. 2005. PMID: 16226581 Review.
Cited by
-
Neuronal composition of processing modules in human V1: laminar density for neuronal and non-neuronal populations and a comparison with macaque.Cereb Cortex. 2024 Jan 31;34(2):bhad512. doi: 10.1093/cercor/bhad512. Cereb Cortex. 2024. PMID: 38183210 Free PMC article.
-
Anisotropy of object nonrigidity: High-level perceptual consequences of cortical anisotropy.bioRxiv [Preprint]. 2025 Feb 15:2024.09.10.612333. doi: 10.1101/2024.09.10.612333. bioRxiv. 2025. PMID: 39345500 Free PMC article. Preprint.
-
Efficient models of cortical activity via local dynamic equilibria and coarse-grained interactions.Proc Natl Acad Sci U S A. 2024 Jul 2;121(27):e2320454121. doi: 10.1073/pnas.2320454121. Epub 2024 Jun 26. Proc Natl Acad Sci U S A. 2024. PMID: 38923983 Free PMC article.
-
Dynamic Recruitment of the Feedforward and Recurrent Mechanism for Black-White Asymmetry in the Primary Visual Cortex.J Neurosci. 2023 Aug 2;43(31):5668-5684. doi: 10.1523/JNEUROSCI.0168-23.2023. Epub 2023 Jul 24. J Neurosci. 2023. PMID: 37487737 Free PMC article.
-
A comprehensive data-driven model of cat primary visual cortex.PLoS Comput Biol. 2024 Aug 21;20(8):e1012342. doi: 10.1371/journal.pcbi.1012342. eCollection 2024 Aug. PLoS Comput Biol. 2024. PMID: 39167628 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
