Epithelial RAC1-dependent cytoskeleton dynamics controls cell mechanics, cell shedding and barrier integrity in intestinal inflammation
- PMID: 35241625
- PMCID: PMC9872254
- DOI: 10.1136/gutjnl-2021-325520
Epithelial RAC1-dependent cytoskeleton dynamics controls cell mechanics, cell shedding and barrier integrity in intestinal inflammation
Abstract
Objective: Increased apoptotic shedding has been linked to intestinal barrier dysfunction and development of inflammatory bowel diseases (IBD). In contrast, physiological cell shedding allows the renewal of the epithelial monolayer without compromising the barrier function. Here, we investigated the role of live cell extrusion in epithelial barrier alterations in IBD.
Design: Taking advantage of conditional GGTase and RAC1 knockout mice in intestinal epithelial cells (Pggt1b iΔIEC and Rac1 iΔIEC mice), intravital microscopy, immunostaining, mechanobiology, organoid techniques and RNA sequencing, we analysed cell shedding alterations within the intestinal epithelium. Moreover, we examined human gut tissue and intestinal organoids from patients with IBD for cell shedding alterations and RAC1 function.
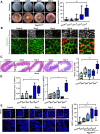
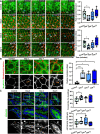
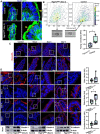
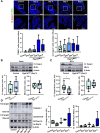
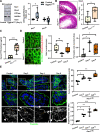
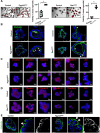
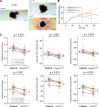
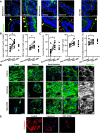
Results: Epithelial Pggt1b deletion led to cytoskeleton rearrangement and tight junction redistribution, causing cell overcrowding due to arresting of cell shedding that finally resulted in epithelial leakage and spontaneous mucosal inflammation in the small and to a lesser extent in the large intestine. Both in vivo and in vitro studies (knockout mice, organoids) identified RAC1 as a GGTase target critically involved in prenylation-dependent cytoskeleton dynamics, cell mechanics and epithelial cell shedding. Moreover, inflamed areas of gut tissue from patients with IBD exhibited funnel-like structures, signs of arrested cell shedding and impaired RAC1 function. RAC1 inhibition in human intestinal organoids caused actin alterations compatible with arresting of cell shedding.
Conclusion: Impaired epithelial RAC1 function causes cell overcrowding and epithelial leakage thus inducing chronic intestinal inflammation. Epithelial RAC1 emerges as key regulator of cytoskeletal dynamics, cell mechanics and intestinal cell shedding. Modulation of RAC1 might be exploited for restoration of epithelial integrity in the gut of patients with IBD.
Keywords: IBD; actin cytoskeleton; epithelial barrier; intestinal epithelium; tight junction.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures






Comment in
-
Epithelial RAC1 niches in IBD: from barrier integrity to cytoskeletal plasticity.Gut. 2023 Feb;72(2):219-220. doi: 10.1136/gutjnl-2022-327089. Epub 2022 Apr 11. Gut. 2023. PMID: 35410889 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
