Proteomic analysis distinguishes extracellular vesicles produced by cancerous versus healthy pancreatic organoids
- PMID: 35241737
- PMCID: PMC8894448
- DOI: 10.1038/s41598-022-07451-6
Proteomic analysis distinguishes extracellular vesicles produced by cancerous versus healthy pancreatic organoids
Abstract
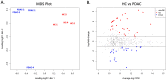
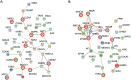
Extracellular vesicles (EVs) are produced and released by both healthy and malignant cells and bear markers indicative of ongoing biological processes. In the present study we utilized high resolution flow cytometry to detect EVs in the plasma of patients with pancreatic ductal adenocarcinoma (PDAC) and in the supernatants of PDAC and healthy control (HC) pancreatic organoid cultures. Using ultrafiltration and size exclusion chromatography, PDAC and HC pancreatic organoid EVs were isolated for mass spectrometry analysis. Proteomic and functional protein network analysis showed a striking distinction in that EV proteins profiled in pancreatic cancer organoids were involved in vesicular transport and tumorigenesis while EV proteins in healthy organoids were involved in cellular homeostasis. Thus, the most abundant proteins identified in either case represented non-overlapping cellular programs. Tumor-promoting candidates LAMA5, SDCBP and TENA were consistently upregulated in PDAC EVs. Validation of specific markers for PDAC EVs versus healthy pancreatic EVs will provide the biomarkers and enhanced sensitivity necessary to monitor early disease or disease progression, with or without treatment. Moreover, disease-associated changes in EV protein profiles provide an opportunity to investigate alterations in cellular programming with disease progression.
© 2022. The Author(s).
Conflict of interest statement
DLM is a consultant for Pfizer, Inc., Alkermes Inc., and Tensive Controls, Inc. DLM receives grant funding and holds equity in Tensive Controls, Inc. All conflicts are reviewed and approved by the OHSU Conflict of Interest Office. All other authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

