Immune response to SARS-CoV-2 after a booster of mRNA-1273: an open-label phase 2 trial
- PMID: 35241844
- PMCID: PMC9117133
- DOI: 10.1038/s41591-022-01739-w
Immune response to SARS-CoV-2 after a booster of mRNA-1273: an open-label phase 2 trial
Abstract
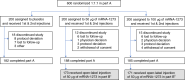
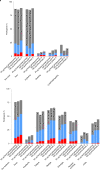
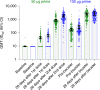
Rising breakthrough infections of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in previously immunized individuals have raised concerns for the need for a booster vaccine dose to combat waning antibody levels and new variants. Here we report the results of the open-label, non-randomized part B of a phase 2 trial in which we evaluated the safety and immunogenicity of a booster injection of 50 µg of the coronavirus disease 2019 (COVID-19) vaccine mRNA-1273 in 344 adult participants immunized 6-8 months earlier with a primary series of two doses of 50 µg or 100 µg of mRNA-1273 ( NCT04405076 ). Neutralizing antibody (nAb) titers against wild-type SARS-CoV-2 at 1 month after the booster were 1.7-fold (95% confidence interval (CI): 1.5, 1.9) higher than those at 28 days after the second injection of the primary series, which met the pre-specified non-inferiority criterion (primary immunogenicity objective) and might indicate a memory B cell response. The nAb titers against the Delta variant (B.1.617.2) (exploratory objective) at 1 month after the booster were 2.1-fold (95% CI: 1.8, 2.4) higher than those at 28 days after the second injection of the primary series. The seroresponse rate (95% CI (four-fold rise from baseline)) was 100% (98.7, 100.0) at 28 days after the booster compared to 98.3% (96.0, 99.4) after the primary series. The higher antibody titers at 28 days after the booster dose compared to 28 days after the second dose in the phase 3 COVE study were also observed in two assays for anti-spike IgG antibody measured by ELISA and by Meso Scale Discovery (MSD) Multiplex. The frequency of solicited local and systemic adverse reactions after the booster dose was similar to that after the second dose in the primary two-dose series of mRNA-1273 (50 µg or 100 µg); no new signals were observed in the unsolicited adverse events; and no serious adverse events were reported in the 1-month follow-up period. These results show that a booster injection of mRNA-1273 more than 6 months after completing the primary two-dose series is safe and elicited nAb titers that were statistically significantly higher than the peak titers detected after the primary vaccination series, suggesting that a booster dose of mRNA-1273 might result in increased vaccine effectiveness against infection and disease caused by SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
W.H., B.N., Y.C., A.C., D.K.E., J.O., H.L., B.G., R.P., J.M.M., R.D., B.L. and R.M. are employees of Moderna and might hold stock/stock options in the company. D.M. has received funding from Moderna for assaying clinical samples. F.J.D. is a Moderna consultant. K.V. declares no competing interests.
Figures
Similar articles
-
Safety and immunogenicity against ancestral, Delta and Omicron virus variants following a booster dose of an inactivated whole-virus COVID-19 vaccine (VLA2001): Interim analysis of an open-label extension of the randomized, controlled, phase 3 COV-COMPARE trial.J Infect. 2023 Sep;87(3):242-254. doi: 10.1016/j.jinf.2023.06.022. Epub 2023 Jul 3. J Infect. 2023. PMID: 37406777 Clinical Trial.
-
Safety, immunogenicity and antibody persistence of a bivalent Beta-containing booster vaccine against COVID-19: a phase 2/3 trial.Nat Med. 2022 Nov;28(11):2388-2397. doi: 10.1038/s41591-022-02031-7. Epub 2022 Oct 6. Nat Med. 2022. PMID: 36202997 Free PMC article. Clinical Trial.
-
Interim safety and immunogenicity of COVID-19 omicron BA.1 variant-containing vaccine in children in the USA: an open-label non-randomised phase 3 trial.Lancet Infect Dis. 2024 Jul;24(7):687-697. doi: 10.1016/S1473-3099(24)00101-4. Epub 2024 Mar 19. Lancet Infect Dis. 2024. PMID: 38518789 Clinical Trial.
-
The effectiveness of the first dose COVID-19 booster vs. full vaccination to prevent SARS-CoV-2 infection and severe COVID-19 clinical event: a meta-analysis and systematic review of longitudinal studies.Front Public Health. 2023 Jun 1;11:1165611. doi: 10.3389/fpubh.2023.1165611. eCollection 2023. Front Public Health. 2023. PMID: 37325336 Free PMC article.
-
Achievements of COVID-19 vaccination programs: Taiwanese perspective.J Formos Med Assoc. 2024 Jan;123 Suppl 1:S70-S76. doi: 10.1016/j.jfma.2023.04.017. Epub 2023 Apr 27. J Formos Med Assoc. 2024. PMID: 37142477 Free PMC article. Review.
Cited by
-
Immune imprinting: The persisting influence of the first antigenic encounter with rapidly evolving viruses.Hum Vaccin Immunother. 2024 Dec 31;20(1):2384192. doi: 10.1080/21645515.2024.2384192. Epub 2024 Aug 16. Hum Vaccin Immunother. 2024. PMID: 39149872 Free PMC article. Review.
-
Systemic and Mucosal Antibody Responses to SARS-CoV-2 Variant-Specific Prime-and-Boost and Prime-and-Spike Vaccination: A Comparison of Intramuscular and Intranasal Bivalent Vaccine Administration in a Murine Model.Vaccines (Basel). 2025 Mar 25;13(4):351. doi: 10.3390/vaccines13040351. Vaccines (Basel). 2025. PMID: 40333249 Free PMC article.
-
Booster dose of mRNA vaccine augments waning T cell and antibody responses against SARS-CoV-2.Front Immunol. 2022 Oct 12;13:1012526. doi: 10.3389/fimmu.2022.1012526. eCollection 2022. Front Immunol. 2022. PMID: 36311732 Free PMC article.
-
Adverse Reactions after Booster SARS-CoV-2 Vaccination Have Less Impact on Antibody Response than after Basic Vaccination Scheme.Vaccines (Basel). 2023 Jan 15;11(1):182. doi: 10.3390/vaccines11010182. Vaccines (Basel). 2023. PMID: 36680026 Free PMC article.
-
Effectiveness of mRNA Vaccine Booster against SARS-CoV-2 Infection and COVID-19 in the Adult Population during the First Three Months of the Omicron Wave in Sicily.Healthcare (Basel). 2023 Jan 19;11(3):305. doi: 10.3390/healthcare11030305. Healthcare (Basel). 2023. PMID: 36766880 Free PMC article.
References
-
- US Food & Drug Administration. FDA Approves First COVID-19 Vaccine. https://www.fda.gov/news-events/press-announcements/fda-approves-first-c... (2021).
-
- US Food & Drug Administration. Moderna COVID-19 Vaccine. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise... (2021).
-
- US Food & Drug Administration. Coronavirus (COVID-19) Update: FDA Takes Multiple Actions to Expand Use of Pfizer-BioNTech COVID-19 Vaccine. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19... (2022).
-
- Ritchie, H. et al. Coronavirus Pandemic (COVID-19). Our World in Data.https://ourworldindata.org/coronavirus
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

