Solid Foam Ru/C Catalysts for Sugar Hydrogenation to Sugar Alcohols-Preparation, Characterization, Activity, and Selectivity
- PMID: 35241873
- PMCID: PMC8883585
- DOI: 10.1021/acs.iecr.1c04501
Solid Foam Ru/C Catalysts for Sugar Hydrogenation to Sugar Alcohols-Preparation, Characterization, Activity, and Selectivity
Abstract
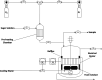
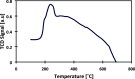
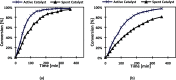
Sugar alcohols are obtained by hydrogenation of sugars in the presence of ruthenium catalysts. The research effort was focused on the development of solid foam catalysts based on ruthenium nanoparticles supported on active carbon. This catalyst was used in kinetic experiments on the hydrogenation of l-arabinose and d-galactose at three temperatures (90, 100, and 120 °C) and two hydrogen pressures (20 and 40 bar). Kinetic experiments were carried out with binary sugar mixtures at different d-galactose-to-l-arabinose molar ratios to study the interactions of these sugars in the presence of the prepared solid foam catalyst. The solid foam catalyst preparation comprised the following steps: cutting of the open-cell foam aluminum pieces, anodic oxidation pretreatment, carbon coating, acid pretreatment, ruthenium incorporation, and ex situ reduction. The carbon coating method comprised the polymerization of furfuryl alcohol, followed by a pyrolysis process and activation with oxygen. Incorporation of ruthenium on the carbon-coated foam was done by incipient wetness impregnation (IWI), using ruthenium(III) nitrosyl nitrate as the precursor. By applying IWI, it was possible to prepare an active catalyst with a ruthenium load of 1.12 wt %, which gave a high conversion of the sugars to the corresponding sugar alcohols. The catalysts were characterized by SEM, HR-TEM, TPR, and ICP-OES to interpret the catalyst behavior in terms of activity, durability, and critical parameters for the catalyst preparation. Extensive kinetic experiments were carried out in an isothermal laboratory-scale semibatch reactor to which gaseous hydrogen was constantly added. High selectivities toward the sugar alcohols, arabitol and galactitol, exceeding 98% were obtained for both sugars, and the sugar conversions were within the range of 53-97%, depending on temperature. The temperature effect on the reaction rate was very strong, while the effect of hydrogen pressure was minor. Regarding the sugar mixtures, in general, l-arabinose presented a higher reaction rate, and an acceleration of the hydrogenation process was observed for both sugars as the ratio of d-galactose to l-arabinose increased, evidently because of competitive interactions on the catalyst surface.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











References
-
- Pinales-Márquez C. D.; Rodríguez-Jasso R. M.; Araújo R. G.; Loredo-Treviño A.; Nabarlatz D.; Gullón B.; Ruiz H. A. Circular Bioeconomy and Integrated Biorefinery in the Production of Xylooligosaccharides from Lignocellulosic Biomass: A Review. Ind. Crops Prod. 2021, 162, 11327410.1016/j.indcrop.2021.113274. - DOI
-
- Zada B.; Chen M.; Chen C.; Yan L.; Xu Q.; Li W.; Guo Q.; Fu Y. Recent Advances in Catalytic Production of Sugar Alcohols and Their Applications. Sci. China: Chem. 2017, 60, 853–869. 10.1007/s11426-017-9067-1. - DOI
-
- Rissanen J. V.; Grénman H.; Willför S.; Murzin D. Y.; Salmi T. Spruce Hemicellulose for Chemicals Using Aqueous Extraction: Kinetics, Mass Transfer, and Modeling. Ind. Eng. Chem. Res. 2014, 53, 6341–6350. 10.1021/ie500234t. - DOI
-
- Ramos-Andrés M.; Aguilera-Torre B.; García-Serna J. Hydrothermal Production of High-Molecular Weight Hemicellulose-Pectin, Free Sugars and Residual Cellulose Pulp from Discarded Carrots. J. Cleaner Prod. 2021, 290, 12517910.1016/j.jclepro.2020.125179. - DOI
LinkOut - more resources
Full Text Sources
