Psychotropic Drug Use in Acute Myeloid Leukaemia (AML) and Myelodysplastic Syndrome (MDS): A Danish Nationwide Matched Cohort Study of 2404 AML and 1307 MDS Patients
- PMID: 35241936
- PMCID: PMC8887140
- DOI: 10.2147/CLEP.S336115
Psychotropic Drug Use in Acute Myeloid Leukaemia (AML) and Myelodysplastic Syndrome (MDS): A Danish Nationwide Matched Cohort Study of 2404 AML and 1307 MDS Patients
Abstract
Introduction: The diagnosis of a life-threatening disease can lead to depression and anxiety resulting in pharmacological treatment. However, use of psychotropic drugs (antidepressants, anxiolytics, and antipsychotics) in acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS) is undetermined.
Methods: Prescription of psychotropic drugs in Danish AML and MDS patients was compared to a cohort matched on age, sex, and country of origin from the Danish background population using national population-based registries.
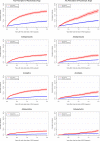
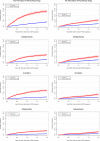
Results: In total, 2404 AML patients (median age 69 years) and 1307 MDS patients (median age 75 years) were included and each matched to five comparators from the background population. Two-year cumulative incidences showed that AML (20.6%) and MDS (21.2%) patients had a high risk of redemption of a psychotropic drug prescription compared to the background population (7.0% and 7.9%). High age, low educational level, and Charlson Comorbidity Index score ≥1 was associated with a higher risk in AML and MDS patients. Furthermore, non-curative treatment intent and performance status in AML patients, and high risk MDS were associated with elevated risk of psychotropic drug prescription.
Conclusion: In conclusion, diagnoses of AML and MDS were associated with a higher rate of psychotropic drugs prescription compared to the background population.
Keywords: acute myeloid leukaemia; anxiety; depression; myelodysplastic syndrome; psychotropic drugs.
© 2022 Jensen et al.
Conflict of interest statement
Dr Andreas Kiesbye Øvlisen reports covered travel expenses from Pfizer and AbbVie, outside the submitted work. Dr Lasse Hjort Jakobsen reports personal fees from Takeda, outside the submitted work. Dr Christian Torp-Pedersen reports grants for randomised study from Bayer, grants for epidemiological study from Novo Nordisk, during the conduct of the study. Professor Tarec Christoffer El-Galaly was previously employed by Roche, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Connect MDS/AML: design of the myelodysplastic syndromes and acute myeloid leukemia disease registry, a prospective observational cohort study.BMC Cancer. 2016 Aug 19;16:652. doi: 10.1186/s12885-016-2710-6. BMC Cancer. 2016. PMID: 27538433 Free PMC article.
-
A review of therapy-related myelodysplastic syndromes and acute myeloid leukaemia (t-MDS/AML) in Irish patients: a single centre experience.Hematology. 2017 Jul;22(6):341-346. doi: 10.1080/10245332.2017.1286539. Epub 2017 Feb 15. Hematology. 2017. PMID: 28196450
-
Second primary non-myeloid malignancies following intensive treatment for adult acute myeloid leukaemia: a Danish population-based cohort study.Lancet Reg Health Eur. 2024 Dec 30;50:101204. doi: 10.1016/j.lanepe.2024.101204. eCollection 2025 Mar. Lancet Reg Health Eur. 2024. PMID: 39844882 Free PMC article.
-
Azacitidine for the treatment of myelodysplastic syndrome, chronic myelomonocytic leukaemia and acute myeloid leukaemia.Health Technol Assess. 2010 May;14 Suppl 1:69-74. doi: 10.3310/hta14Suppl1/10. Health Technol Assess. 2010. PMID: 20507806 Review.
-
The Danish National Acute Leukemia Registry.Clin Epidemiol. 2016 Oct 25;8:553-560. doi: 10.2147/CLEP.S99460. eCollection 2016. Clin Epidemiol. 2016. PMID: 27822099 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

