Emerging Role of Translational Digital Biomarkers Within Home Cage Monitoring Technologies in Preclinical Drug Discovery and Development
- PMID: 35242017
- PMCID: PMC8885444
- DOI: 10.3389/fnbeh.2021.758274
Emerging Role of Translational Digital Biomarkers Within Home Cage Monitoring Technologies in Preclinical Drug Discovery and Development
Abstract
In drug discovery and development, traditional assessment of human patients and preclinical subjects occurs at limited time points in potentially stressful surroundings (i.e., the clinic or a test arena), which can impact data quality and welfare. However, recent advances in remote digital monitoring technologies enable the assessment of human patients and preclinical subjects across multiple time points in familiar surroundings. The ability to monitor a patient throughout disease progression provides an opportunity for more relevant and efficient diagnosis as well as improved assessment of drug efficacy and safety. In preclinical in vivo animal models, these digital technologies allow for continuous, longitudinal, and non-invasive monitoring in the home environment. This manuscript provides an overview of digital monitoring technologies for use in preclinical studies including their history and evolution, current engagement through use cases, and impact of digital biomarkers (DBs) on drug discovery and the 3Rs. We also discuss barriers to implementation and strategies to overcome them. Finally, we address data consistency and technology standards from the perspective of technology providers, end-users, and subject matter experts. Overall, this review establishes an improved understanding of the value and implementation of digital biomarker (DB) technologies in preclinical research.
Keywords: 3Rs (reduce replace refine); digital biomarkers; drug discovery and development; home cage; preclinical; rodents; translation.
Copyright © 2022 Baran, Bratcher, Dennis, Gaburro, Karlsson, Maguire, Makidon, Noldus, Potier, Rosati, Ruiter, Schaevitz, Sweeney and LaFollette.
Conflict of interest statement
SB is employed by Novartis Pharmaceuticals Corporation. NB and PM are employed by AbbVie Inc. NB and PM also own AbbVie stock. SG and GR are employed by Tecniplast S.p.A. EK is employed by Calico Life Sciences LLC. SM is employed by GlaxoSmithKline USA. LN is employed by Noldus Information Technology BV. YP is employed by Tessera Therapeutics Inc. MR is employed by Unified Information Devices Inc. LS is employed by Recursion Pharmaceuticals Inc. PS is employed by Actual Analytics Ltd and Naason Science Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
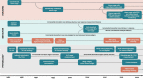




References
-
- Balcombe J. P., Barnard N. D., Sandusky C. (2004). Laboratory routines cause animal stress. Contemporary Topics in Laboratory Animal Science 43 42–51. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

