Dynamics of Neuromuscular Transmission Reproduced by Calcium-Dependent and Reversible Serial Transitions in the Vesicle Fusion Complex
- PMID: 35242023
- PMCID: PMC8885725
- DOI: 10.3389/fnsyn.2021.785361
Dynamics of Neuromuscular Transmission Reproduced by Calcium-Dependent and Reversible Serial Transitions in the Vesicle Fusion Complex
Abstract
Neuromuscular transmission, from spontaneous release to facilitation and depression, was accurately reproduced by a mechanistic kinetic model of sequential maturation transitions in the molecular fusion complex. The model incorporates three predictions. First, calcium-dependent forward transitions take vesicles from docked to preprimed to primed states, followed by fusion. Second, prepriming and priming are reversible. Third, fusion and recycling are unidirectional. The model was fed with experimental data from previous studies, whereas the backward (β) and recycling (ρ) rate constant values were fitted. Classical experiments were successfully reproduced with four transition states in the model when every forward (α) rate constant had the same value, and both backward rate constants were 50-100 times larger. Such disproportion originated an abruptly decreasing gradient of resting vesicles from docked to primed states. By contrast, a three-state version of the model failed to reproduce the dynamics of transmission by using the same set of parameters. Simulations predict the following: (1) Spontaneous release reflects primed to fusion spontaneous transitions. (2) Calcium elevations synchronize the series of forward transitions that lead to fusion. (3) Facilitation reflects a transient increase of priming following the calcium-dependent maturation transitions. (4) The calcium sensors that produce facilitation are those that evoke the transitions form docked to primed states. (5) Backward transitions and recycling restore the resting state. (6) Depression reflects backward transitions and slow recycling after intense release. Altogether, our results predict that fusion is produced by one calcium sensor, whereas the modulation of the number of vesicles that fuse depends on the calcium sensors that promote the early transition states. Such finely tuned kinetics offers a mechanism for collective non-linear transitional adaptations of a homogeneous vesicle pool to the ever-changing pattern of electrical activity in the neuromuscular junction.
Keywords: calcium; depression; facilitation; fusion complex; kinetics; neuromuscular synapse; synapse; transmitter release.
Copyright © 2022 Martínez-Valencia, Ramírez-Santiago and De-Miguel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
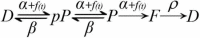



Similar articles
-
Rapid regulation of vesicle priming explains synaptic facilitation despite heterogeneous vesicle:Ca2+ channel distances.Elife. 2020 Feb 20;9:e51032. doi: 10.7554/eLife.51032. Elife. 2020. PMID: 32077852 Free PMC article.
-
Complexin Mutants Reveal Partial Segregation between Recycling Pathways That Drive Evoked and Spontaneous Neurotransmission.J Neurosci. 2017 Jan 11;37(2):383-396. doi: 10.1523/JNEUROSCI.1854-16.2016. J Neurosci. 2017. PMID: 28077717 Free PMC article.
-
A sequential vesicle pool model with a single release sensor and a Ca(2+)-dependent priming catalyst effectively explains Ca(2+)-dependent properties of neurosecretion.PLoS Comput Biol. 2013;9(12):e1003362. doi: 10.1371/journal.pcbi.1003362. Epub 2013 Dec 5. PLoS Comput Biol. 2013. PMID: 24339761 Free PMC article.
-
Synaptic Vesicles Having Large Contact Areas with the Presynaptic Membrane are Preferentially Hemifused at Active Zones of Frog Neuromuscular Junctions Fixed during Synaptic Activity.Int J Mol Sci. 2019 May 31;20(11):2692. doi: 10.3390/ijms20112692. Int J Mol Sci. 2019. PMID: 31159267 Free PMC article. Review.
-
Dynamically Primed Synaptic Vesicle States: Key to Understand Synaptic Short-Term Plasticity.Neuron. 2018 Dec 19;100(6):1283-1291. doi: 10.1016/j.neuron.2018.11.024. Neuron. 2018. PMID: 30571941 Review.
Cited by
-
Structure and Function of the Mammalian Neuromuscular Junction.Compr Physiol. 2022 Aug 11;12(4):3731-3766. doi: 10.1002/cphy.c210022. Compr Physiol. 2022. PMID: 35950651 Free PMC article.
-
A sequential two-step priming scheme reproduces diversity in synaptic strength and short-term plasticity.Proc Natl Acad Sci U S A. 2022 Aug 23;119(34):e2207987119. doi: 10.1073/pnas.2207987119. Epub 2022 Aug 15. Proc Natl Acad Sci U S A. 2022. PMID: 35969787 Free PMC article.
References
LinkOut - more resources
Full Text Sources

