Oxidase enzymes as sustainable oxidation catalysts
- PMID: 35242351
- PMCID: PMC8753158
- DOI: 10.1098/rsos.211572
Oxidase enzymes as sustainable oxidation catalysts
Abstract
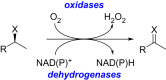
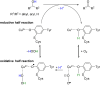
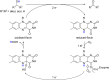
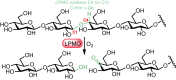
Oxidation is one of the most important processes used by the chemical industry. However, many of the methods that are used pose significant sustainability and environmental issues. Biocatalytic oxidation offers an alternative to these methods, with a now significant enzymatic oxidation toolbox on offer to chemists. Oxidases are one of these options, and as they only depend on molecular oxygen as a terminal oxidant offer perfect atom economy alongside the selectivity benefits afforded by enzymes. This review will focus on examples of oxidase biocatalysts that have been used for the sustainable production of important molecules and highlight some important processes that have been significantly improved through the use of oxidases. It will also consider emerging classes of oxidases, and how they might fit in a future biorefinery approach for the sustainable production of important chemicals.
Keywords: alcohol oxidation; amine oxidation; biocatalysis; oxidases; sustainable oxidation.
© 2022 The Authors.
Conflict of interest statement
We have no competing interests.
Figures
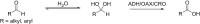


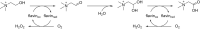
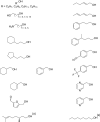

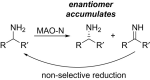
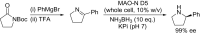
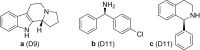
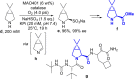
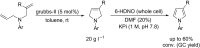
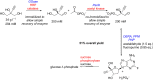
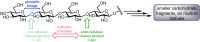

References
-
- de Nooy AEJ, Besemer AC, van Bekkum H. 1995. Selective oxidation of primary alcohols mediated by nitroxyl radical in aqueous solution. Kinetics and mechanism. Tetrahedron 51, 8023-8032. (10.1016/0040-4020(95)00417-7) - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

