Experience with denosumab (XGEVA®) for prevention of skeletal-related events in the 10 years after approval
- PMID: 35242510
- PMCID: PMC8857591
- DOI: 10.1016/j.jbo.2022.100416
Experience with denosumab (XGEVA®) for prevention of skeletal-related events in the 10 years after approval
Abstract
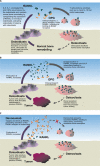
Skeletal-related events (SREs) are complications of bone metastases and carry a significant patient and economic burden. Denosumab is a receptor activator of nuclear factor-κB ligand (RANKL) inhibitor approved for SRE prevention in patients with multiple myeloma and patients with bone metastases from solid tumors. In phase 3 trials, denosumab showed superiority to the bisphosphonate zoledronate in reducing the risk of first on-study SRE by 17% (median time to first on-study SRE delayed by 8.2 months) and the risk of first and subsequent on-study SREs by 18% across multiple solid tumor types, including some patients with multiple myeloma. Denosumab also improved pain outcomes and reduced the need for strong opioids. Additionally, a phase 3 trial showed denosumab was noninferior to zoledronate in delaying time to first SRE in patients with newly diagnosed multiple myeloma. Denosumab has a convenient 120 mg every 4 weeks recommended dosing schedule with subcutaneous administration. Rare but serious toxicities associated with denosumab include osteonecrosis of the jaw, hypocalcemia, and atypical femoral fracture events, with multiple vertebral fractures reported following treatment discontinuation. After a decade of real-world clinical experience with denosumab, we are still learning about the optimal use and dosing for denosumab. Despite the emergence of novel and effective antitumor therapies, there remains a strong rationale for the clinical utility of antiresorptive therapy for SRE prevention. Ongoing studies aim to optimize clinical management of patients using denosumab for SRE prevention while maintaining safety and efficacy.
Keywords: AFF, Atypical femoral fracture; BM, Bone metastasis; BMFS, BM-free survival; BP, Bisphosphonate; BTA, Bone-targeting agent; Bone metastasis; CI, Confidence interval; Denosumab; Efficacy; HR, Hazard ratio; HRQoL, Health-related quality of life; IMWG, International Myeloma Working Group; MM, Multiple myeloma; MVF, Multiple vertebral fracture; NSCLC, Non–small-cell lung cancer; ONJ, Osteonecrosis of the jaw; OPG, Osteoprotegerin; OS, Overall survival; PFS, Progression-free survival; Q12W, Every 12 weeks; Q4W, Every 4 weeks; RANKL, Receptor activator of nuclear factor-κB ligand; SC, Subcutaneous; SRE, Skeletal-related event; Safety; Skeletal-related events; uNTx/Cr, Urinary N-telopeptide normalized to urinary creatinine.
© 2022 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Benoit Cadieux was an employee and shareholder of Amgen Inc. at the time of the development of this review. Robert Coleman has received lecture fees from Amgen and Novartis; consultancy fees from Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, ITM, and Scancell. Pegah Jafarinasabian is an employee and shareholder of Amgen Inc. Allan Lipton has no financial interests or personal relationships that may be considered as potential competing interests. Robert Z. Orlowski has no financial interests or personal relationships that may be considered as potential competing interests.. Fred Saad served as a consultant, advisory board member and received honoraria and research funding from Amgen; he has also served as a consultant, advisory board member and received honoraria and research funding from Astellas, AstraZeneca, Bayer, Janssen, Myovant, Novartis, Pfizer, and Sanofi. Giorgio V. Scagliotti has no financial interests or personal relationships that may be considered as potential competing interests. Kazuyuki Shimiz has no financial interests or personal relationships that may be considered as potential competing interests. Alison Stopeck has received consulting fees from Amgen and AstraZeneca; contracted research support from Amgen, Exact Sciences; speakers bureau from Exact Sciences; and honoraria from Amgen.
Figures

References
-
- von Moos R., Body J.-J., Egerdie B., Stopeck A., Brown J., Fallowfield L., Patrick D.L., Cleeland C., Damyanov D., Palazzo F.S., Marx G., Zhou Y., Braun A., Balakumaran A., Qian Y.i. Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support. Care Cancer. 2016;24(3):1327–1337. - PMC - PubMed
-
- Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12(20):6243s–6249s. - PubMed
-
- Coleman R.E., Croucher P.I., Padhani A.R., Clezardin P., Chow E., Fallon M., Guise T., Colangeli S., Capanna R., Costa L. Bone metastases. Nat. Rev. Dis. Primers. 2020;6(1):83. - PubMed

