Giant cell tumor of bone: A single center study of 115 cases
- PMID: 35242511
- PMCID: PMC8881473
- DOI: 10.1016/j.jbo.2022.100417
Giant cell tumor of bone: A single center study of 115 cases
Abstract
Background: Giant cell tumor of bone (GCTB) is a locally aggressive bone tumor that represents about 4-5% of all primary bone tumors. It is characterized by aggressive growth, possible recurrence after surgical treatment and, in rare cases, metastasis. Surgical management is the primary treatment and may include intralesional curettage with adjuvants or, in rare cases, wide resection. In recent years the monoclonal antibody denosumab has been introduced as a potential (neo-)adjuvant systemic treatment option for patients with borderline resectable or unresectable lesions. Currently several studies reported that the use of denosumab prior to curettage possibly increase the risk of local recurrence.
Methods: In this retrospective study we reviewed 115 cases of GCT with a mean follow-up of 65.6 (24-404) months who underwent a surgical treatment with or without preoperative denosumab therapy in our institution. Potential risk factors for LR and complications were analyzed.
Results: The study includes 47 male (40.9%) and 68 female (59.1%) patients with a mean age of 33.9 (10-77) years and a mean follow-up of 65.6 (24-404) months. Denosumab was used in 33 (28.7%) cases, in 14 cases (12.2%) in a neoadjuvant setting and in 17 cases preoperatively before re-curettage (14.8%) after LR. In 105 cases (91.3%) an intralesional curettage was performed. The overall LR rate was 47.8% (55 cases). Patients who underwent intralesional curettage and bone cement augmentation without neoadjuvant denosumab treatment had LR in 42.2% (38/90) of the cases. Patients who underwent neoadjuvant denosumab treatment prior to curettage had LR in 28.6% (4/14). Re-recurrence was frequent in patients with neoadjuvant denosumab treatment who had LR after initial curettage (50%, 8/16). After wide resection and endoprosthetic replacement one case (20%) of local recurrence was detectable (1/5 cases).
Conclusions: GCTB recurs frequently after intralesional curettage and cement augmentation. While denosumab is a potential (neo-)adjuvant treatment option that might be used for lesions that are difficult to resect, surgeons should be aware that LR is still frequent.
Keywords: Denosumab; GCTB, Giant cell tumor of bone; Giant cell tumor; Giant cell tumor of bone; Intralesional curettage.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

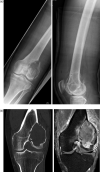


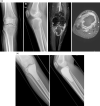

Similar articles
-
Preoperative Denosumab With Curettage and Cryotherapy in Giant Cell Tumor of Bone: Is There an Increased Risk of Local Recurrence?Clin Orthop Relat Res. 2018 Sep;476(9):1783-1790. doi: 10.1007/s11999.0000000000000104. Clin Orthop Relat Res. 2018. PMID: 30778015 Free PMC article.
-
Evaluation of Local Recurrence in Giant-Cell Tumor of Bone Treated by Neoadjuvant Denosumab.Clin Orthop Surg. 2019 Sep;11(3):352-360. doi: 10.4055/cios.2019.11.3.352. Epub 2019 Aug 12. Clin Orthop Surg. 2019. PMID: 31475058 Free PMC article.
-
Preoperative denosumab treatment with curettage may be a risk factor for recurrence of giant cell tumor of bone.J Orthop Surg (Hong Kong). 2020 Jan-Apr;28(2):2309499020929786. doi: 10.1177/2309499020929786. J Orthop Surg (Hong Kong). 2020. PMID: 32539628
-
Role of (Neo)adjuvant Denosumab for Giant Cell Tumor of Bone.Curr Treat Options Oncol. 2020 Jul 4;21(8):68. doi: 10.1007/s11864-020-00766-4. Curr Treat Options Oncol. 2020. PMID: 32623530 Review.
-
Is Treatment with Denosumab Associated with Local Recurrence in Patients with Giant Cell Tumor of Bone Treated with Curettage? A Systematic Review.Clin Orthop Relat Res. 2020 May;478(5):1076-1085. doi: 10.1097/CORR.0000000000001074. Clin Orthop Relat Res. 2020. PMID: 31794487 Free PMC article.
Cited by
-
Progress on Denosumab Use in Giant Cell Tumor of Bone: Dose and Duration of Therapy.Cancers (Basel). 2022 Nov 23;14(23):5758. doi: 10.3390/cancers14235758. Cancers (Basel). 2022. PMID: 36497239 Free PMC article. Review.
-
Non‑surgical outcomes and risk factors for pulmonary metastasis from giant cell tumor of bone.Oncol Lett. 2023 Oct 11;26(6):508. doi: 10.3892/ol.2023.14095. eCollection 2023 Dec. Oncol Lett. 2023. PMID: 37920440 Free PMC article.
-
Denosumab for giant cell tumors of bone from 2010 to 2022: a bibliometric analysis.Clin Exp Med. 2023 Nov;23(7):3053-3075. doi: 10.1007/s10238-023-01079-0. Epub 2023 Apr 27. Clin Exp Med. 2023. PMID: 37103655 Review.
-
Management of RANKL-mediated Disorders With Denosumab in Children and Adolescents: A Global Expert Guidance Document.J Clin Endocrinol Metab. 2024 Apr 19;109(5):1371-1382. doi: 10.1210/clinem/dgad657. J Clin Endocrinol Metab. 2024. PMID: 38041865 Free PMC article.
-
Current Concepts in the Treatment of Giant Cell Tumor of Bone: An Update.Curr Oncol. 2024 Apr 8;31(4):2112-2132. doi: 10.3390/curroncol31040157. Curr Oncol. 2024. PMID: 38668060 Free PMC article. Review.
References
-
- Balke M., Schremper L., Gebert C., Ahrens H., Streitbuerger A., Koehler G., Hardes J., Gosheger G. Giant cell tumor of bone: treatment and outcome of 214 cases. J. Cancer Res. Clin. Oncol. 2008;134(9):969–978. - PubMed
-
- Liede A., Bach B.A., Stryker S., Hernandez R.K., Sobocki P., Bennett B., Wong S.S. Regional variation and challenges in estimating the incidence of giant cell tumor of bone. J. Bone Joint Surg. Am. 2014;96(23):1999–2007. - PubMed
-
- Chan C.M., Adler Z., Reith J.D., Gibbs C.P., Jr. Risk factors for pulmonary metastases from giant cell tumor of bone. J. Bone Joint Surg. Am. 2015;97(5):420–428. - PubMed
-
- Campanacci M., Baldini N., Boriani S., Sudanese A. Giant-cell tumor of bone. J. Bone Joint Surg. Am. 1987;69(1):106–114. - PubMed
LinkOut - more resources
Full Text Sources

