Heterologous production of chondroitin
- PMID: 35242620
- PMCID: PMC8858990
- DOI: 10.1016/j.btre.2022.e00710
Heterologous production of chondroitin
Abstract
Chondroitin sulfate (CS) is a glycosaminoglycan with a broad range of applications being a popular dietary supplement for osteoarthritis. Usually, CS is extracted from animal sources. However, the known risks of animal products use have been driving the search for alternative methods and sources to obtain this compound. Several pathogenic bacteria naturally produce chondroitin-like polysaccharides through well-known pathways and, therefore, have been the basis for numerous studies that aim to produce chondroitin using non-pathogenic hosts. However, the yields obtained are not enough to meet the high demand for this glycosaminoglycan. Metabolic engineering strategies have been used to construct improved heterologous hosts. The identification of metabolic bottlenecks and regulation points, and the screening for efficient enzymes are key points for constructing microbial cell factories with improved chondroitin yields to achieve industrial CS production. The recent advances on enzymatic and microbial strategies to produce non-animal chondroitin are herein reviewed. Challenges and prospects for future research are also discussed.
Keywords: Biosynthetic pathway; Chondroitin; Glycosaminoglycans; Heterologous production; Metabolic engineering; Microbial fermentation.
© 2022 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
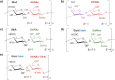
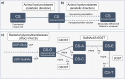

References
-
- Casale J., Crane J.S. StatPearls Publishing; Treasure Island (FL): 2021. Biochemistry, Glycosaminoglycans.http://www.ncbi.nlm.nih.gov/pubmed/31335015 (accessed July 4, 2021) - PubMed
-
- A. Varki, R.D. Cummings, J.D. Esko, P. Stanley, G.W. Hart, M. Aebi, A.G. Darvill, T. Kinoshita, N.H. Packer, J.H. Prestegard, R.L. Schnaar, P.H. Seeberger, Essentials of Glycobiology, Cold Spring Harb. (2017) 823. https://www.ncbi.nlm.nih.gov/books/NBK310274/ (accessed July 24, 2021). - PubMed
-
- Kovensky J., Grand E., Uhrig M.L. In: Ind. Appl. Renew. Biomass Prod. Goyanes S.N., D'Accorso N.B., editors. Springer International Publishing; Cham: 2017. Applications of glycosaminoglycans in the medical, veterinary, pharmaceutical, and cosmetic fields; pp. 135–164. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
