Effects of delayed HIF-1α expression in astrocytes on myelination following hypoxia-ischaemia white matter injury in immature rats
- PMID: 35242649
- PMCID: PMC8825930
- DOI: 10.21037/tp-21-407
Effects of delayed HIF-1α expression in astrocytes on myelination following hypoxia-ischaemia white matter injury in immature rats
Abstract
Background: The underlying cause of neurological sequelae after immature cerebral hypoxia-ischaemia (HI) white matter injury is impaired myelination. Previous studies have indicated that astrocyte activation is closely related to impaired myelination. However, the mechanism of reactive gliosis in white matter injury post-HI remains poorly understood.
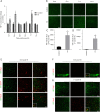
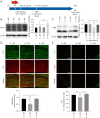
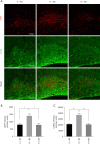
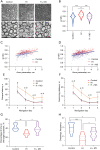
Methods: Studies using adult ischaemic animal models demonstrated that hypoxia inducible factor-1α (HIF-1α) expression was involved in the formation of reactive astrocytes. Here, we investigated the temporal expression of HIF-1α and its impact on reactive gliosis and further myelination using a perinatal HI white matter injury model induced in rats at postnatal day 3. The temporal pattern of HIF-1α expression post-HI injury was tested by western blotting and immunofluorescence. Rats were treated with a HIF-1α inhibitor at 72 hours post-HI injury. Reactive gliosis and myelination were assessed with western blotting, immunofluorescence and electron microscopy, and neurological functions were examined by behavioural testing.
Results: Our results showed that the expression of HIF-1α was upregulated in neurons at 24 hours and in astrocytes at 7 days post-HI. Inhibiting delayed HIF-1α expression post-HI injury could restrain reactive gliosis, ameliorate hypomyelination, and improve the performance of rats in the Morris water maze test.
Conclusions: Our findings suggest that a delayed increase in HIF-1α in astrocytes is involved in glial scar formation and leads to arrested oligodendrocyte maturation, impaired myelination, and long-term neurological function after experimental white matter injury in immature rats.
Keywords: White matter injury; astrogliosis; hypoxia inducible factor-1α (HIF-1α); myelination; oligodendrocytes.
2022 Translational Pediatrics. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-21-407/coif). The authors have no conflicts of interest to declare.
Figures

References
-
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736-88. 10.1016/S0140-6736(18)32203-7 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
