Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus
- PMID: 35242721
- PMCID: PMC8886906
- DOI: 10.3389/fcimb.2022.834485
Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus
Abstract
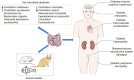
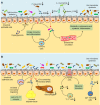
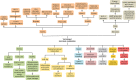
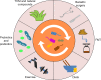
Type 2 diabetes mellitus (T2DM) is one of the common metabolic diseases in the world. Due to the rise in morbidity and mortality, it has become a global health problem. To date, T2DM still cannot be cured, and its intervention measures mainly focus on glucose control as well as the prevention and treatment of related complications. Interestingly, the gut microbiota plays an important role in the development of metabolic diseases, especially T2DM. In this review, we introduce the characteristics of the gut microbiota in T2DM population, T2DM animal models, and diabetic complications. In addition, we describe the molecular mechanisms linking host and the gut microbiota in T2DM, including the host molecules that induce gut microbiota dysbiosis, immune and inflammatory responses, and gut microbial metabolites involved in pathogenesis. These findings suggest that we can treat T2DM and its complications by remodeling the gut microbiota through interventions such as drugs, probiotics, prebiotics, fecal microbiota transplantation (FMT) and diets.
Keywords: glucose metabolism; gut microbiota; insulin resistance; pathogenesis; type 2 diabetes mellitus.
Copyright © 2022 Zhou, Sun, Yu and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

