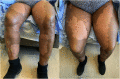
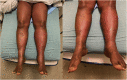
Severe cutaneous drug toxicity following enfortumab vedotin treatment for metastatic urothelial carcinoma
- PMID: 35242967
- PMCID: PMC8857547
- DOI: 10.1016/j.jdcr.2022.01.005
Severe cutaneous drug toxicity following enfortumab vedotin treatment for metastatic urothelial carcinoma
Keywords: EV, enfortumab vedotin; MMAE, monomethyl auristatin E; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; UC, urothelial carcinoma; cutaneous drug toxicity; enfortumab vedotin; exfoliative dermatitis; general dermatology.
Conflict of interest statement
None disclosed.
Figures


References
-
- Viscuse P.V., Marques-Piubelli M.L., Heberton M.M., et al. Case report: Enfortumab vedotin for metastatic urothelial carcinoma: a case series on the clinical and histopathologic spectrum of adverse cutaneous reactions from fatal Stevens-Johnson syndrome/toxic epidermal necrolysis to dermal hypersensitivity reaction. Front Oncol. 2021;11:621591. doi: 10.3389/fonc.2021.621591. - DOI - PMC - PubMed
-
- Wu S., Adamson A.S. Cutaneous toxicity associated with enfortumab vedotin treatment of metastatic urothelial carcinoma. Dermatol Online J. 2019;25(2):13030–qt4j44w7w6. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
