Safety and effectiveness of recombinant factor XIII-A2 in congenital factor XIII deficiency: Real-world evidence
- PMID: 35243202
- PMCID: PMC8882239
- DOI: 10.1002/rth2.12628
Safety and effectiveness of recombinant factor XIII-A2 in congenital factor XIII deficiency: Real-world evidence
Abstract
Background: Regular factor XIII (FXIII) prophylaxis is standard treatment for congenital FXIII A-subunit deficiency (FXIII-A CD). Recombinant factor XIII-A2 (rFXIII-A2) was extensively evaluated in the mentor trials.
Objective: To assess real-world safety and treatment effectiveness of rFXIII-A2 prophylaxis from the mentor 6 trial.
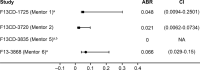
Patients/methods: mentor 6 was a noninterventional, postauthorization safety study investigating rFXIII-A2 prophylaxis in FXIII-A CD. rFXIII-A2 treatment was observed for 2 to 6 years per patient. The primary end point was documentation of adverse drug reactions (including anti-FXIII antibody development). Secondary end points were serious adverse events (SAEs), medical events of special interest (MESIs), and annualized bleeding rate (ABR).
Results: Among 30 patients (mean age, 25.5 years), there were 44 adverse events (AEs) (30 mild, 13 moderate, 1 severe). Eleven AEs were possibly/probably related to rFXIII-A2. Of four MESIs, two were unlikely related to rFXIII-A2 (accidental overdose, deep vein thrombosis), and two were possibly/probably related (nonneutralizing anti-FXIII antibody, decreased therapeutic response). All 10 SAEs were unlikely related to rFXIII-A2. Over a follow-up of 75.4 patient-years, there were six treatment-requiring bleeds (all trauma-related with no spontaneous bleeds), giving a treatment-requiring ABR of 0.066; five bleeds were treated successfully with rFXIII-A2. Eight of nine minor surgeries performed during rFXIII-A2 prophylaxis reported successful hemostatic outcomes (one missing evaluation).
Conclusions: These data confirm that rFXIII-A2 prophylaxis is well tolerated as long-term care. There were no spontaneous bleeds, ABR was low, and rFXIII-A2 successfully treated bleeds in patients receiving rFXIII-A2 prophylaxis.
Keywords: factor XIII; long‐term care; recombinant factor XIII‐A2; safety; treatment effectiveness.
© 2022 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Figures
References
-
- Bolton‐Maggs PH, Perry DJ, Chalmers EA, et al. The rare coagulation disorders–review with guidelines for management from the United Kingdom Haemophilia Centre Doctors’ Organisation. Haemophilia. 2004;10:593‐628. - PubMed
-
- Carcao M, Fukutake K, Inbal A, et al. Developing the first recombinant factor XIII for congenital factor XIII deficiency: clinical challenges and successes. Semin Thromb Hemost. 2017;43:59‐68. - PubMed
-
- Inbal A, Oldenburg J, Carcao M, Rosholm A, Tehranchi R, Nugent D. Recombinant factor XIII: a safe and novel treatment for congenital factor XIII deficiency. Blood. 2012;119:5111‐5117. - PubMed
-
- Lovejoy AE, Reynolds TC, Visich JE, et al. Safety and pharmacokinetics of recombinant factor XIII‐A2 administration in patients with congenital factor XIII deficiency. Blood. 2006;108:57‐62. - PubMed
-
- Menegatti M, Palla R, Boscarino M, et al. Minimal factor XIII activity level to prevent major spontaneous bleeds. J Thromb Haemost. 2017;15:1728‐1736. - PubMed
LinkOut - more resources
Full Text Sources

