The KRAS-G12D mutation induces metabolic vulnerability in B-cell acute lymphoblastic leukemia
- PMID: 35243242
- PMCID: PMC8861657
- DOI: 10.1016/j.isci.2022.103881
The KRAS-G12D mutation induces metabolic vulnerability in B-cell acute lymphoblastic leukemia
Abstract
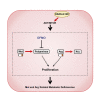
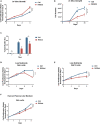
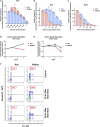
Mutations in RAS pathway genes are highly prevalent in acute lymphoblastic leukemia (ALL). However, the effects of RAS mutations on ALL cell growth have not been experimentally characterized, and effective RAS-targeting therapies are being sought after. Here, we found that Reh ALL cells bearing the KRAS-G12D mutation showed increased proliferation rates in vitro but displayed severely compromised growth in mice. Exploring this divergence, proliferation assays with multiple ALL cell lines revealed that the KRAS-G12D rewired methionine and arginine metabolism. Isotope tracing results showed that KRAS-G12D promotes catabolism of methionine and arginine to support anabolism of polyamines and proline, respectively. Chemical inhibition of polyamine biosynthesis selectively killed KRAS-G12D B-ALL cells. Finally, chemically inhibiting AKT/mTOR signaling abrogated the altered amino acid metabolism and strongly promoted the in vivo growth of KRAS-G12D cells in B-ALL xenograft. Our study thus illustrates how hyperactivated AKT/mTOR signaling exerts distinct impacts on hematological malignancies vs. solid tumors.
Keywords: Biochemistry; Biological sciences; Molecular biology.
© 2022 The Author(s).
Conflict of interest statement
All authors declare no competing interests. Z.K. is an employee of Hangzhou Calibra dignostic, Ltd., and declares no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

