Altered inhibitory function in hippocampal CA2 contributes in social memory deficits in Alzheimer's mouse model
- PMID: 35243253
- PMCID: PMC8873612
- DOI: 10.1016/j.isci.2022.103895
Altered inhibitory function in hippocampal CA2 contributes in social memory deficits in Alzheimer's mouse model
Abstract
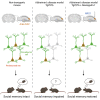
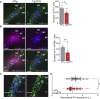
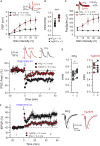
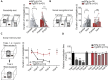
Parvalbumin (PV)-expressing interneurons which are often associated with the specific extracellular matrix perineuronal net (PNN) play a critical role in the alteration of brain activity and memory performance in Alzheimer's disease (AD). The integrity of these neurons is crucial for normal functioning of the hippocampal subfield CA2, and hence, social memory formation. Here, we find that social memory deficits of mouse models of AD are associated with decreased presence of PNN around PV cells and long-term synaptic plasticity in area CA2. Furthermore, single local injection of the growth factor neuregulin-1 (NRG1) is sufficient to restore both PV/PNN levels and social memory performance of these mice. Thus, the PV/PNN disruption in area CA2 could play a causal role in social memory deficits of AD mice, and activating PV cell pro-maturation pathways may be sufficient to restore social memory.
Keywords: Behavioral neuroscience; Cellular neuroscience; Molecular neuroscience.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
-
- Cattaud V., Bezzina C., Rey C.C., Lejards C., Dahan L., Verret L. Early disruption of parvalbumin expression and perineuronal nets in the hippocampus of the Tg2576 mouse model of Alzheimer’s disease can be rescued by enriched environment. Neurobiol. Aging. 2018;72:147–158. doi: 10.1016/j.neurobiolaging.2018.08.024. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

