Unlocking a self-catalytic cycle in a copper-catalyzed aerobic oxidative coupling/cyclization reaction
- PMID: 35243259
- PMCID: PMC8881718
- DOI: 10.1016/j.isci.2022.103906
Unlocking a self-catalytic cycle in a copper-catalyzed aerobic oxidative coupling/cyclization reaction
Abstract
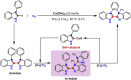
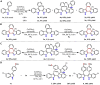
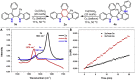
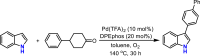
Presented here is a copper-catalyzed, aerobic oxidative C-H/C-H cyclization reaction, which occurs by cleaving the C-H and N-H bonds of 3-phenylindoles. A broad range of 3-phenylindoles can be well tolerated to produce the indole-containing polycyclic aromatic hydrocarbons (PAH) in good to excellent yields. An evaluation of the reaction mechanism is enabled by the isolation of the di- and tri-indole intermediates, highlighting the role of the substrate for this catalytic reaction. The results of these controlled experiments and kinetic studies provide solid experimental support for a self-catalysis reaction, which has rarely been observed in oxidative C-H activation reactions. Additional mechanistic studies indicate that the substrate for this reaction accelerates by the following mechanism: The substrate combines with the Cu catalyst to transform the less active di-indole intermediate into a tri-indole intermediate. This intermediate is quickly converted into the desired product along with regeneration of the substrate copper complex.
Keywords: Catalysis; Chemical engineering; Chemistry.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures












References
-
- Ackermann L., Wang L., Lygin A.V. Ruthenium-catalyzed aerobic oxidative coupling of alkynes with 2-aryl-substituted pyrroles. Chem. Sci. 2012;3:177–180.
-
- Bissette A.J., Fletcher S.P. Mechanisms of autocatalysis. Angew. Chem. Int. Ed. 2013;52:12800–12826. - PubMed
-
- Bachmann J., Zierold R., Chong Y.T., Hauert R., Sturm C., Schmidt-Grund R., Rheinländer B., Grundmann M., Gösele U., Nielsch K. A practical, self-catalytic, atomic layer deposition of silicon dioxide. Angew. Chem. Int. Ed. 2008;47:6177–6179. - PubMed
LinkOut - more resources
Full Text Sources

