Using long-range freeze-preventive vaccine carriers in Nepal: A study of equipment performance, acceptability, systems fit, and cost
- PMID: 35243322
- PMCID: PMC8867128
- DOI: 10.1016/j.jvacx.2022.100146
Using long-range freeze-preventive vaccine carriers in Nepal: A study of equipment performance, acceptability, systems fit, and cost
Abstract
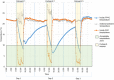
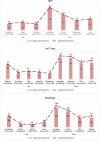
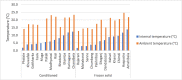
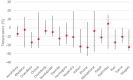
Preventing vaccine freezing is one of the biggest challenges in vaccine management. Until 2018, vaccine carriers used in the immunization program lacked features to prevent vaccine freezing. Freeze-preventive vaccine carriers (FPVCs) have an engineered liner that buffers vaccines from direct exposure to frozen ice packs. A field evaluation of three FPVCs was conducted in 24 health posts in eastern Nepal. The objective was to evaluate the FPVCs' performance, acceptability, systems fit, and cost, to inform prequalification and introduction planning. The study was carried out in two phases: in the first phase, FPVCs containing dummy vaccines (labeled "Not for Human Use") were transported to outreach sessions along with a standard vaccine carrier (SVC); in the second phase, the FPVCs were used for transporting vaccines taken to outreach sessions and used for vaccinating eligible children. The study gathered quantitative and qualitative data from health workers, logbooks, and electronic temperature monitors placed inside and outside the FPVCs. Results indicate the FPVCs successfully prevented temperatures below 0 °C more than 99% of the time-except at one site, where ambient temperatures were below the minimum rated testing temperature specified by the World Health Organization. Internal cool-down times for the FPVCs were highly variable, as were mean kinetic temperatures, possibly driven by the wide range of ambient temperatures and higher-than-expected variations in freezer performance, which, along with the need to transport ice packs to some locations, affected ice-pack temperatures. Almost all health workers requested smaller, lighter-weight FPVCs but appreciated the FPVCs' ability to prevent vaccines from freezing while avoiding undue heat exposure. FPVCs had benefit-cost ratios greater than 1 and hence good value for money. Results point to the importance of understanding the intended environment of use and the need for smaller, short-range as well as long-range carriers.
Keywords: AHW, auxiliary health worker; ANM, auxiliary nurse midwife; BPKIHS, B.P. Koirala Institute of Health Sciences; CCH, cold chain handler; Cold chain equipment; FPVC, freeze-preventive vaccine carrier; Freeze-preventive vaccine carrier; HP, health post; Immunization; Innovation; MKT, mean kinetic temperature; MOHP, Ministry of Health and Population; N/A, not applicable; PQS, Performance, Quality and Safety; SVC, standard vaccine carrier; VVM, vaccine vial monitor; Vaccine cold chain; Vaccine freezing; WHO, World Health Organization.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- World Health Organization (WHO). Preventing freeze-damage to vaccines. Aide-memoire for prevention of freeze damage to vaccines. Geneva: WHO; 2019. <ext-link xmlns:xlink="http://www.w3.org/1999/xlink" ext-link-type="uri" xlink:href="https://apps.who.int/iris/bitstream/handle/10665/69673/WHO_IVB_07.09_eng..."><underline>https://apps.who.int/iris/bitstream/handle/10665/69673/WHO_IVB_07.09_eng...</underline></ext-link>.
-
- World Health Organization (WHO). Guidelines on the international packaging and shipping of vaccines. Geneva: WHO; 2005. <ext-link xmlns:xlink="http://www.w3.org/1999/xlink" ext-link-type="uri" xlink:href="https://apps.who.int/iris/bitstream/handle/10665/69368/WHO_IVB_05.23_eng..."><underline>https://apps.who.int/iris/bitstream/handle/10665/69368/WHO_IVB_05.23_eng...</underline></ext-link>
-
- Techathawat S, Varinsathien P, Rasdjarmrearnsook A, Tharmaphornpilas P. Exposure to heat and freezing in the vaccine cold chain in Thailand. Vaccine 2007;25:1328–33. . - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

