The future of inhalation therapy in chronic obstructive pulmonary disease
- PMID: 35243334
- PMCID: PMC8866667
- DOI: 10.1016/j.crphar.2022.100092
The future of inhalation therapy in chronic obstructive pulmonary disease
Abstract
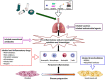
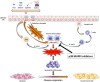
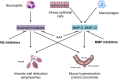
The inhaled route is critical for the administration of drugs to treat patients suffering from COPD, but there is still an unmet need for new and innovative inhalers to address some limitations of existing products that do not make them suitable for many COPD patients. The treatment of COPD, currently limited to the use of bronchodilators, corticosteroids, and antibiotics, requires a significant expansion of the therapeutic armamentarium that is closely linked to the widening of knowledge on the pathogenesis and evolution of COPD. The great interest in the development of new drugs that may be able to interfere in the natural history of the disease is leading to the synthesis of numerous new molecules, of which however only a few have entered the stages of clinical development. On the other hand, further improvement of inhaled drug delivery could be an interesting possibility because it targets the organ of interest directly, requires significantly less drug to exert the pharmacological effect and, by lowering the amount of drug needed, reduces the cost of therapy. Unfortunately, however, the development of new inhaled drugs for use in COPD is currently too slow.
Keywords: Anti-inflammatory drugs; Antimicrobial agents; Bronchodilators; COPD; Inhalation route; Inhaler devices; Monoclonal antibodies; Vaccines.
© 2022 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: M Cazzola was a faculty member and advisor in scientific meetings sponsored by Abdi Ibrahim, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Cipla, Edmond Pharma, GlaxoSmithKline, Glenmark, Lallemand, Menarini Group, Mundipharma, Novartis, Pfizer, Teva, Verona Pharma, and Zambon, and is or was a consultant to ABC Farmaceutici, AstraZeneca, Chiesi Farmaceutici, Edmond Pharma, Lallemand, Novartis, Ockham Biotech, VeronaPharma, and Zambon. J Ora reported personal fees from AstraZeneca and has participated as a speaker in scientific meetings sponsored by AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Novartis, and Zambon. L Calzetta participated as an advisor in scientific meetings sponsored by Boehringer Ingelheim and Novartis, received non-financial support from AstraZeneca, a research grant partially funded by Chiesi Farmaceutici, Boehringer Ingelheim, Novartis, and Almirall, and is or was a consultant to ABC Farmaceutici, Recipharm, Zambon, Verona Pharma and Ockham Biotech. His department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis and Zambon. P Rogliani reported grants and personal fees from Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis, and participated as a lecturer and advisor in scientific meetings sponsored by Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, Edmond Pharma, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis. Her department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis, and Zambon. MG Matera participated as a faculty member and advisor in scientific meetings sponsored by ABC Farmaceutici, Almirall, AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, and Novartis, and was a consultant to Chiesi Farmaceutici, and GlaxoSmithKline. Her department was funded by GlaxoSmithKline, and Novartis.
Figures



Similar articles
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Overuse of long-acting β2-agonist/inhaled corticosteroids in patients with chronic obstructive pulmonary disease: time to rethink prescribing patterns.Postgrad Med. 2023 Nov;135(8):784-802. doi: 10.1080/00325481.2023.2284650. Epub 2024 Jan 10. Postgrad Med. 2023. PMID: 38032494 Review.
-
The pharmacological management of asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS).Expert Opin Pharmacother. 2020 Feb;21(2):213-231. doi: 10.1080/14656566.2019.1701656. Expert Opin Pharmacother. 2020. PMID: 31955671 Review.
-
Delivery technology of inhaled therapy for asthma and COPD.Adv Pharmacol. 2023;98:273-311. doi: 10.1016/bs.apha.2023.03.001. Epub 2023 Mar 28. Adv Pharmacol. 2023. PMID: 37524490
-
Use of pressurized metered dose inhalers in patients with chronic obstructive pulmonary disease: review of evidence.Expert Rev Respir Med. 2014 Jun;8(3):349-56. doi: 10.1586/17476348.2014.905916. Epub 2014 May 7. Expert Rev Respir Med. 2014. PMID: 24802511 Review.
Cited by
-
Digital health technologies for improving the management of people with chronic obstructive pulmonary disease.Front Digit Health. 2025 Aug 6;7:1640585. doi: 10.3389/fdgth.2025.1640585. eCollection 2025. Front Digit Health. 2025. PMID: 40843141 Free PMC article. Review.
-
Pathophysiology, Therapeutic Targets, and Future Therapeutic Alternatives in COPD: Focus on the Importance of the Cholinergic System.Biomolecules. 2023 Mar 5;13(3):476. doi: 10.3390/biom13030476. Biomolecules. 2023. PMID: 36979411 Free PMC article. Review.
-
Perceptions of the effectiveness of non-pharmacological management of respiratory disorders among CRD patients.Medicine (Baltimore). 2023 Oct 13;102(41):e35474. doi: 10.1097/MD.0000000000035474. Medicine (Baltimore). 2023. PMID: 37832130 Free PMC article.
-
Recalibrating Perceptions and Attitudes Toward Nebulizers versus Inhalers for Maintenance Therapy in COPD: Past as Prologue.Int J Chron Obstruct Pulmon Dis. 2024 Nov 28;19:2571-2586. doi: 10.2147/COPD.S491275. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 39629181 Free PMC article. Review.
-
RETRACTED: Involvement of GPR43 Receptor in Effect of Lacticaseibacillus rhamnosus on Murine Steroid Resistant Chronic Obstructive Pulmonary Disease: Relevance to Pro-Inflammatory Mediators and Oxidative Stress in Human Macrophages.Nutrients. 2024 May 16;16(10):1509. doi: 10.3390/nu16101509. Nutrients. 2024. Retraction in: Nutrients. 2025 Jul 31;17(15):2513. doi: 10.3390/nu17152513. PMID: 38794746 Free PMC article. Retracted.
References
-
- Abbott-Banner K.H., Page C.P. Dual PDE3/4 and PDE4 inhibitors: novel treatments for COPD and other inflammatory airway diseases. Basic Clin. Pharmacol. Toxicol. 2014;114(5):365–376. - PubMed
-
- Ambery C., Young G., Fuller T., Georgiou A., Ramsay D., Puri A., Daley-Yates P. Open-label, crossover study to determine the pharmacokinetics of fluticasone furoate and batefenterol when administered alone, in combination, or concurrently. Clin. Pharmacol. Drug Dev. 2019;8(2):188–197. - PMC - PubMed
-
- Ari A., Fink J.B. Recent advances in aerosol devices for the delivery of inhaled medications. Expet Opin. Drug Deliv. 2020;17(2):133–144. - PubMed
-
- Bao W., Li Y., Wang T., Li X., He J., Wang Y., et al. Effects of influenza vaccination on clinical outcomes of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ageing Res. Rev. 2021;68 - PubMed
-
- Barth P., Bruijnzeel P., Wach A., Sellier Kessler O., Hooftman L., Zimmermann J., et al. Single dose escalation studies with inhaled POL6014, a potent novel selective reversible inhibitor of human neutrophil elastase, in healthy volunteers and subjects with cystic fibrosis. J. Cyst. Fibros. 2020;19(2):299–304. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

