Dysfunction of parvalbumin-expressing cells in the thalamic reticular nucleus induces cortical spike-and-wave discharges and an unconscious state
- PMID: 35243344
- PMCID: PMC8887905
- DOI: 10.1093/braincomms/fcac010
Dysfunction of parvalbumin-expressing cells in the thalamic reticular nucleus induces cortical spike-and-wave discharges and an unconscious state
Abstract
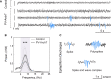
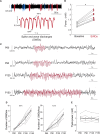
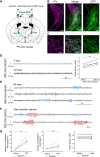
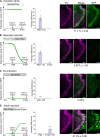
Spike-and-wave discharges and an accompanying loss of consciousness are hallmarks of absence seizure, which is a childhood generalized epilepsy disorder. In absence seizure, dysfunction of the cortico-thalamo-cortico circuitry is thought to engage in abnormal cortical rhythms. Previous studies demonstrated that the thalamic reticular nucleus has a critical role in the formation of normal cortical rhythms; however, whether thalamic reticular nucleus dysfunction leads directly to abnormal rhythms, such as epilepsy, is largely unknown. We found that expressing the inhibitory opsin, archaerhodopsin, including in the thalamic reticular nucleus, caused abnormal cortical rhythms in Pvalb-tetracycline transactivator::tetO-ArchT (PV-ArchT) double transgenic mice. We validated the PV-ArchT line as a new mouse model of absence seizure through physiological and pharmacological analyses, as well as through examining their behavioural features. We then discovered that archaerhodopsin expression exclusively in thalamic reticular nucleus parvalbumin-positive neurons was sufficient to induce cortical spike-and-wave discharges using adeno-associated virus-mediated thalamic reticular nucleus targeting. Furthermore, we found that archaerhodopsin expression impaired rebound burst firing and T-current in thalamic reticular nucleus parvalbumin-positive cells by slice physiology. Although T-current in the thalamic reticular nucleus was impaired, the T-current blocker ethosuximide still had a therapeutic effect in PV-ArchT mice, suggesting a gain of function of T-type calcium channels in this absence seizure model. However, we did not find any over- or misexpression of T-type calcium channel genes in the thalamus or the cortex. Thus, we demonstrated that thalamic reticular nucleus dysfunction led to an absence seizure-like phenotype in mice. In a final set of experiments, we showed that the archaerhodopsin-mediated absence seizure-like phenotype disappeared after the removal of archaerhodopsin by using a time-controllable transgenic system. These data may provide a hint as to why many absence seizures naturally regress.
Keywords: T-type calcium channel; animal model of absence seizure; inhibitory opsin; rebound burst firing; tetracycline-controllable gene induction.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures





Similar articles
-
Temporal and Potential Predictive Relationships between Sleep Spindle Density and Spike-and-Wave Discharges.eNeuro. 2024 Sep 18;11(9):ENEURO.0058-24.2024. doi: 10.1523/ENEURO.0058-24.2024. Print 2024 Sep. eNeuro. 2024. PMID: 39256042 Free PMC article.
-
Isolated P/Q Calcium Channel Deletion in Layer VI Corticothalamic Neurons Generates Absence Epilepsy.J Neurosci. 2016 Jan 13;36(2):405-18. doi: 10.1523/JNEUROSCI.2555-15.2016. J Neurosci. 2016. PMID: 26758833 Free PMC article.
-
Cortical and Thalamic PV+ Interneuron Dysfunction in the Pathogenesis of Absence Epilepsy.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 21. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 21. PMID: 39637158 Free Books & Documents. Review.
-
The impact of silencing feed-forward parvalbumin-expressing inhibitory interneurons in the cortico-thalamocortical network on seizure generation and behaviour.Neurobiol Dis. 2019 Dec;132:104610. doi: 10.1016/j.nbd.2019.104610. Epub 2019 Sep 5. Neurobiol Dis. 2019. PMID: 31494287
-
Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures.Brain. 2020 Aug 1;143(8):2341-2368. doi: 10.1093/brain/awaa072. Brain. 2020. PMID: 32437558 Free PMC article. Review.
Cited by
-
Reticular thalamic hyperexcitability drives autism spectrum disorder behaviors in the Cntnap2 model of autism.Sci Adv. 2025 Aug 22;11(34):eadw4682. doi: 10.1126/sciadv.adw4682. Epub 2025 Aug 20. Sci Adv. 2025. PMID: 40834072 Free PMC article.
-
Progressive generalized tonic-clonic seizures in a transgenic mouse model of adult-onset epilepsy: Implications for morphological changes in cortico-limbic and brainstem structures.Epilepsy Res. 2023 Aug;194:107178. doi: 10.1016/j.eplepsyres.2023.107178. Epub 2023 Jun 2. Epilepsy Res. 2023. PMID: 37295319 Free PMC article.
-
Mediodorsal thalamic nucleus mediates resistance to ethanol through Cav3.1 T-type Ca2+ regulation of neural activity.Elife. 2025 Jul 18;13:RP93200. doi: 10.7554/eLife.93200. Elife. 2025. PMID: 40679136 Free PMC article.
-
Different Alterations of Hippocampal and Reticulo-Thalamic GABAergic Parvalbumin-Expressing Interneurons Underlie Different States of Unconsciousness.Int J Mol Sci. 2023 Apr 5;24(7):6769. doi: 10.3390/ijms24076769. Int J Mol Sci. 2023. PMID: 37047741 Free PMC article.
-
Temporal and Potential Predictive Relationships between Sleep Spindle Density and Spike-and-Wave Discharges.eNeuro. 2024 Sep 18;11(9):ENEURO.0058-24.2024. doi: 10.1523/ENEURO.0058-24.2024. Print 2024 Sep. eNeuro. 2024. PMID: 39256042 Free PMC article.
References
-
- Fisher RS. The new classification of seizures by the International League Against Epilepsy 2017. Curr Neurol Neurosci Rep. 2017;17(6):48. - PubMed
-
- Akman O, Demiralp T, Ates N, Onat FY. Electroencephalographic differences between WAG/Rij and GAERS rat models of absence epilepsy. Epilepsy Res. 2010;89(2-3):185–193. - PubMed
-
- Crunelli V, Leresche N. Childhood absence epilepsy: Genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3(5):371–382. - PubMed
-
- Snead Iii OC. Basic mechanisms of generalized absence seizures. Ann Neurol. 1995;37(2):146–157. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
