The STAR care pathway for patients with pain at 3 months after total knee replacement: a multicentre, pragmatic, randomised, controlled trial
- PMID: 35243362
- PMCID: PMC8873030
- DOI: 10.1016/S2665-9913(21)00371-4
The STAR care pathway for patients with pain at 3 months after total knee replacement: a multicentre, pragmatic, randomised, controlled trial
Erratum in
-
Correction to Lancet Rheumatol 2022; 4: e188-97.Lancet Rheumatol. 2023 May 8;5(6):e314. doi: 10.1016/S2665-9913(23)00130-3. eCollection 2023 Jun. Lancet Rheumatol. 2023. PMID: 37228806 Free PMC article.
Abstract
Background: Approximately 20% of people experience chronic pain after total knee replacement, but effective treatments are not available. We aimed to evaluate the clinical effectiveness and cost-effectiveness of a new care pathway for chronic pain after total knee replacement.
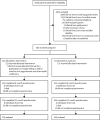
Methods: We did an unmasked, parallel group, pragmatic, superiority, randomised, controlled trial at eight UK National Health Service (NHS) hospitals. People with chronic pain at 3 months after total knee replacement surgery were randomly assigned (2:1) to the Support and Treatment After Replacement (STAR) care pathway plus usual care, or to usual care alone. The STAR intervention aimed to identify underlying causes of chronic pain and enable onward referrals for targeted treatment through a 3-month post-surgery assessment with an extended scope practitioner and telephone follow-up over 12 months. Co-primary outcomes were self-reported pain severity and pain interference in the replaced knee, assessed with the Brief Pain Inventory (BPI) pain severity and interference scales at 12 months (scored 0-10, best to worst) and analysed on an as-randomised basis. Resource use, collected from electronic hospital records and participants, was valued with UK reference costs. Quality-adjusted life-years (QALYs) were calculated from EQ-5D-5L responses. This trial is registered with ISRCTN, ISRCTN92545361.
Findings: Between Sept 6, 2016, and May 31, 2019, 363 participants were randomly assigned to receive the intervention plus usual care (n=242) or to receive usual care alone (n=121). Participants had a median age of 67 years (IQR 61 to 73), 217 (60%) of 363 were female, and 335 (92%) were White. 313 (86%) patients provided follow-up data at 12 months after randomisation (213 assigned to the intervention plus usual care and 100 assigned to usual care alone). At 12 months, the mean between-group difference in the BPI severity score was -0·65 (95% CI -1·17 to -0·13; p=0·014) and the mean between-group difference in the BPI interference score was -0·68 (-1·29 to -0·08; p=0·026), both favouring the intervention. From an NHS and personal social services perspective, the intervention was cost-effective (greater improvement with lower cost), with an incremental net monetary benefit of £1256 (95% CI 164 to 2348) at £20 000 per QALY threshold. One adverse reaction of participant distress was reported in the intervention group.
Interpretation: STAR is a clinically effective and cost-effective intervention to improve pain outcomes over 1 year for people with chronic pain at 3 months after total knee replacement surgery.
Funding: National Institute for Health Research.
© 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Conflict of interest statement
AWB and VW have received funding from Stryker for research unrelated to this work. DAW has received grant funding from Pfizer; consulting fees from Pfizer, AbbVie, GlaxoSmithKline, Reckitt- Benckiser, Galapagos, and EliLilly; and payment for lectures, presentations, and educational events from Pfizer and AbbVie, all unrelated to this work. All other authors declare no competing interests.
Figures

Similar articles
-
The STAR care pathway for patients with chronic pain after total knee replacement: four-year follow-up of a randomised controlled trial.BMC Musculoskelet Disord. 2023 Dec 16;24(1):972. doi: 10.1186/s12891-023-07099-x. BMC Musculoskelet Disord. 2023. PMID: 38102656 Free PMC article. Clinical Trial.
-
Better post-operative prediction and management of chronic pain in adults after total knee replacement: the multidisciplinary STAR research programme including RCT.Southampton (UK): National Institute for Health and Care Research; 2023 Jun. Southampton (UK): National Institute for Health and Care Research; 2023 Jun. PMID: 37494508 Free Books & Documents. Review.
-
Clinical- and cost-effectiveness of the STAR care pathway compared to usual care for patients with chronic pain after total knee replacement: study protocol for a UK randomised controlled trial.Trials. 2018 Feb 21;19(1):132. doi: 10.1186/s13063-018-2516-8. Trials. 2018. PMID: 29467019 Free PMC article.
-
Modelled cost-effectiveness analysis of the Support and Treatment After Replacement (STAR) care pathway for chronic pain after total knee replacement compared with usual care.Cost Eff Resour Alloc. 2024 Apr 11;22(1):28. doi: 10.1186/s12962-024-00532-5. Cost Eff Resour Alloc. 2024. PMID: 38605347 Free PMC article.
-
Infection after total joint replacement of the hip and knee: research programme including the INFORM RCT [Internet].Southampton (UK): National Institute for Health and Care Research; 2022 Nov. Southampton (UK): National Institute for Health and Care Research; 2022 Nov. PMID: 36469653 Free Books & Documents. Review.
Cited by
-
Perioperative Opioid Use and Dosage Trajectories Vary Depending on Pain Outcome Classification and Bodily Pain in Patients who Catastrophize About Their Pain: A Secondary Analysis of a Randomized Trial in Knee Arthroplasty.J Pain. 2024 May;25(5):104434. doi: 10.1016/j.jpain.2023.11.017. Epub 2023 Nov 23. J Pain. 2024. PMID: 38007035 Free PMC article. Clinical Trial.
-
Pre-operative education and prehabilitation provision for patients undergoing hip and knee replacement: a national survey of current NHS practice.BMC Musculoskelet Disord. 2025 Apr 29;26(1):421. doi: 10.1186/s12891-025-08637-5. BMC Musculoskelet Disord. 2025. PMID: 40301783 Free PMC article.
-
The STAR care pathway for patients with chronic pain after total knee replacement: four-year follow-up of a randomised controlled trial.BMC Musculoskelet Disord. 2023 Dec 16;24(1):972. doi: 10.1186/s12891-023-07099-x. BMC Musculoskelet Disord. 2023. PMID: 38102656 Free PMC article. Clinical Trial.
-
Experiences of recovery and a new care pathway for people with pain after total knee replacement: qualitative research embedded in the STAR trial.BMC Musculoskelet Disord. 2022 May 13;23(1):451. doi: 10.1186/s12891-022-05423-5. BMC Musculoskelet Disord. 2022. PMID: 35562815 Free PMC article. Clinical Trial.
-
Practical Application of Value-Based Medicine in Chronic Pelvic Pain: A Qualitative Study.J Eval Clin Pract. 2025 Feb;31(1):e14295. doi: 10.1111/jep.14295. J Eval Clin Pract. 2025. PMID: 39733252 Free PMC article.
References
-
- National Joint Registry; Hertfordshire: 2020. 17th Annual report 2020. - PubMed
-
- Werner MU, Kongsgaard UEI. I. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113:1–4. - PubMed
-
- Jeffery AE, Wylde V, Blom AW, Horwood JP. “It's there and I'm stuck with it”: patients' experiences of chronic pain following total knee replacement surgery. Arthritis Care Res. 2011;63:286–292. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

