Development of an adverse outcome pathway for intrahepatic cholestasis of pregnancy
- PMID: 35243364
- PMCID: PMC8885608
- DOI: 10.1016/j.crtox.2022.100065
Development of an adverse outcome pathway for intrahepatic cholestasis of pregnancy
Abstract
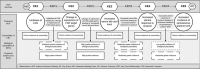
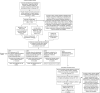
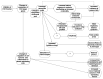
Adverse Outcome Pathways (AOPs) are a research synthesis tool, used primarily by toxicologists for numerous applications including: hypothesis generation, data integration, biomarker determination, and identification of gaps in current knowledge. The AOP model provides a means for evaluating critical interactions between stressors and biological systems which result in adversity, meaning there is significant potential value in using this model in clinical research. However, AOPs have so far not been applied in this context, which may be attributable to the fact that the method is not yet streamlined with established practices in evidence-based medicine, such as systematic review. Here, we present one approach to developing a clinically focused AOP for intrahepatic cholestasis of pregnancy; aiming to enhance understanding of the mechanistic link between this common, gestational liver disease and its association with preterm birth. Mechanistic aspects of the disease pathogenesis, and use of AOPs to broaden inclusion and improve integration of in vitro and in vivo data in clinical research are discussed. We also demonstrate for the first time how central components of systematic review can be integrated into the development of an AOP.
Keywords: Adverse outcome pathway; Cholestasis; Pregnancy; Reproductive medicine.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Abu‐Hayyeh S., Papacleovoulou G., Lövgren‐Sandblom A., Tahir M., Oduwole O., Jamaludin N.A., Ravat S., Nikolova V., Chambers J., Selden C., Rees M., Marschall H.-U., Parker M.G., Williamson C. Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype. Hepatology. 2013;57(2):716–726. - PMC - PubMed
-
- Ahanya S.N., Lakshmanan J., Morgan B.L.G., Ross M.G. Meconium passage in utero: mechanisms, consequences and management. Obstet. Gynecol. Surv. 2005;60(1):45–56. - PubMed
-
- Alsulyman O.M., Ouzounian J.G., Ames-Castro M., et al. Intrahepatic cholestasis of pregnancy: perinatal outcome associated with expectant management. Am. J. Obstetr. Gynecol. 1996;175(4,1):957–960. - PubMed
-
- Barth A., Klinger G., Rost M. Influence of ethinyloestradiol propanolsulphonate on serum bile acids in healthy volunteers. Exp. Toxicol. Pathol. 2003;54(5-6):381–386. - PubMed
LinkOut - more resources
Full Text Sources

