Rate of permanent cardiac implantable electronic device infections after active fixation temporary transvenous pacing: A nationwide Danish cohort study
- PMID: 35243435
- PMCID: PMC8859779
- DOI: 10.1016/j.hroo.2021.11.008
Rate of permanent cardiac implantable electronic device infections after active fixation temporary transvenous pacing: A nationwide Danish cohort study
Abstract
Background: Temporary transvenous pacing (TP) has been associated with an increased risk of cardiac implantable electronic device (CIED) infections, but there is little data to document this in contemporary populations.
Objective: To investigate the impact of active fixation TP on rate of CIED infections in a nationwide cohort of Danish patients.
Methods: We identified all patients who underwent a first-time CIED implantation between 2009 and 2017. Patients were categorized according to TP status at implantation and followed for 1 year. The primary outcome was local or systemic CIED infection resulting in device system removal. The secondary outcomes were systemic CIED infections and hospitalization for infective endocarditis (IE).
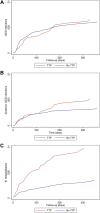
Results: We included a total of 40,601 CIED patients. A total of 2952 were treated with active fixation TP. The primary outcome was met in 246 patients. Risk of CIED infection at 1 year was 0.61% for patients not treated with TP and 0.65% for patients who were, HR of 1.28 (95% CI 0.80-2.05) and adjusted HR 0.85 (95% CI 0.51-1.42). More systemic CIED infections and IE hospitalizations occurred in TP patients; however, these differences did not persist after confounder adjustment. Cumulative mortality at 1 year was 16.8% in patients with TP vs 8.4% in patients without.
Conclusion: Active fixation TP was not associated with a higher rate of CIED infections. Patients treated with TP had higher mortality, more systemic CIED infections, and more IE hospitalizations within first year of implantation. Most was attributable to an accumulation of risk factors for infection among TP patients.
Keywords: Cardiac implantable electronic device; Complications; Epidemiology; Infection; Infective endocarditis; Pacemaker; Temporary transvenous pacing.
© 2021 Heart Rhythm Society. Published by Elsevier Inc.
Figures
References
-
- Glikson M., Nielsen J.C., Kronborg M.B., et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) with the special contribution of the European Heart Rhythm Association (EHRA) Europace. 2021 doi: 10.1093/europace/euab232. Accessed December 18, 2021. - DOI
-
- Blomstrom-Lundqvist C., Traykov V., Erba P.A., et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2020;57:e1–e31. - PubMed
-
- Kusumoto F.M., Schoenfeld M.H., Wilkoff B.L., et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551. - PubMed
-
- Metkus T.S., Schulman S.P., Marine J.E., Eid S.M. Complications and outcomes of temporary transvenous pacing: an analysis of > 360,000 patients from the national inpatient sample. Chest. 2019;155:749–757. - PubMed
-
- de Cock C.C., Van Campen C.M., In't Veld J.A., Visser C.A. Utility and safety of prolonged temporary transvenous pacing using an active-fixation lead: comparison with a conventional lead. Pacing Clin Electrophysiol. 2003;26:1245–1248. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

