The wing imaginal disc
- PMID: 35243513
- PMCID: PMC8982031
- DOI: 10.1093/genetics/iyac020
The wing imaginal disc
Abstract
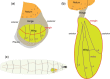
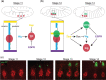
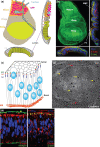
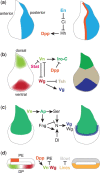
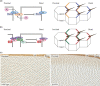
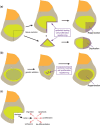
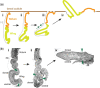
The Drosophila wing imaginal disc is a tissue of undifferentiated cells that are precursors of the wing and most of the notum of the adult fly. The wing disc first forms during embryogenesis from a cluster of ∼30 cells located in the second thoracic segment, which invaginate to form a sac-like structure. They undergo extensive proliferation during larval stages to form a mature larval wing disc of ∼35,000 cells. During this time, distinct cell fates are assigned to different regions, and the wing disc develops a complex morphology. Finally, during pupal stages the wing disc undergoes morphogenetic processes and then differentiates to form the adult wing and notum. While the bulk of the wing disc comprises epithelial cells, it also includes neurons and glia, and is associated with tracheal cells and muscle precursor cells. The relative simplicity and accessibility of the wing disc, combined with the wealth of genetic tools available in Drosophila, have combined to make it a premier system for identifying genes and deciphering systems that play crucial roles in animal development. Studies in wing imaginal discs have made key contributions to many areas of biology, including tissue patterning, signal transduction, growth control, regeneration, planar cell polarity, morphogenesis, and tissue mechanics.
Keywords: Drosophila; FlyBook; imaginal disc; wing.
© The Author(s) 2022. Published by Oxford University Press on behalf of Genetics Society of America.
Figures



References
-
- Adler PN, Charlton J, Liu J.. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125(5):959–968. - PubMed
-
- Aegerter-Wilmsen T, Aegerter CM, Hafen E, Basler K.. Model for the regulation of size in the wing imaginal disc of Drosophila. Mech Dev. 2007;124(4):318–326. - PubMed
-
- Aegerter-Wilmsen T, Heimlicher MB, Smith AC, de Reuille PB, Smith RS, Aegerter CM, Basler K.. Integrating force-sensing and signaling pathways in a model for the regulation of wing imaginal disc size. Development. 2012;139(17):3221–3231. - PubMed
-
- Agnès F, Suzanne M, Noselli S.. The Drosophila JNK pathway controls the morphogenesis of imaginal discs during metamorphosis. Development. 1999;126(23):5453–5462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

