Therapeutic progress and challenges for triple negative breast cancer: targeted therapy and immunotherapy
- PMID: 35243562
- PMCID: PMC8894518
- DOI: 10.1186/s43556-022-00071-6
Therapeutic progress and challenges for triple negative breast cancer: targeted therapy and immunotherapy
Abstract
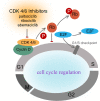
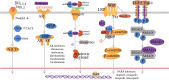
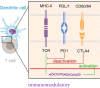
Triple negative breast cancer (TNBC) is a subtype of breast cancer, with estrogen receptor, human epidermal growth factor receptor 2 and progesterone receptor negative. TNBC is characterized by high heterogeneity, high rates of metastasis, poor prognosis, and lack of therapeutic targets. Now the treatment of TNBC is still based on surgery and chemotherapy, which is effective only in initial stage but almost useless in advanced stage. And due to the lack of hormone target, hormonal therapies have little beneficial effects. In recent years, signaling pathways and receptor-specific targets have been reported to be effective in TNBC patients under specific clinical conditions. Now targeted therapies have been approved for many other cancers and even other subtypes of breast cancer, but treatment options for TNBC are still limited. Most of TNBC patients showed no response, which may be related to the heterogeneity of TNBC, therefore more effective treatments and predictive biomarkers are needed. In the present review, we summarize potential treatment opinions for TNBC based on the dysregulated receptors and signaling pathways, which play a significant role in multiple stages of TNBC development. We also focus on the application of immunotherapy in TNBC, and summarize the preclinical and clinical trials of therapy for patients with TNBC. We hope to accelerate the research and development of new drugs for TNBC by understanding the relevant mechanisms, and to improve survival.
Keywords: Immune checkpoint inhibitors; Signaling pathways; TNBC; Targeted therapy; Triple negative breast cancer.
© 2022. The Author(s).
Conflict of interest statement
All authors have declared that no competing interest exists.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
