Severe Acute Respiratory Syndrome Coronavirus 2 Third Vaccine Immune Response in Multiple Sclerosis Patients Treated with Ocrelizumab
- PMID: 35243687
- PMCID: PMC9082479
- DOI: 10.1002/ana.26343
Severe Acute Respiratory Syndrome Coronavirus 2 Third Vaccine Immune Response in Multiple Sclerosis Patients Treated with Ocrelizumab
Abstract
The introduction of a third-dose vaccination along with new variants of concern raises questions regarding serology and T-cell responses in patients with multiple sclerosis (pwMS) treated with B-cell depletion who develop attenuated humoral response to vaccines. The aim of this study was to longitudinally evaluate humoral and cellular response to SARS-CoV-2 mRNA vaccine in ocrelizumab-treated pwMS before and following a third vaccine dose. Following the third vaccine dose, patients who are low or nonresponders following initial vaccination did not increase antibody titers. In healthy controls and ocrelizumab-treated pwMS, cellular response decreased 6 months after initial vaccination and increased significantly after the third dose. ANN NEUROL 2022;91:796-800.
© 2022 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
C.R. is an employee and shareholder of F. Hoffmann‐La Roche. A.V.‐D. reported grants from F. Hoffmann‐La Roche during the conduct of the study.
Figures
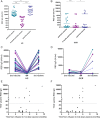
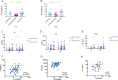
References
-
- Sabatino JJ, Mittl K, Rowles W, et al. Impact of multiple sclerosis disease‐modifying therapies on SARS‐CoV‐2 vaccine‐induced antibody and T cell immunity. medRxiv 2021; 2021.09.10.21262933.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

