Epigallocatechin gallate triggers apoptosis by suppressing de novo lipogenesis in colorectal carcinoma cells
- PMID: 35243817
- PMCID: PMC9063442
- DOI: 10.1002/2211-5463.13391
Epigallocatechin gallate triggers apoptosis by suppressing de novo lipogenesis in colorectal carcinoma cells
Abstract
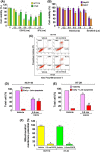
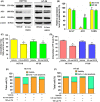
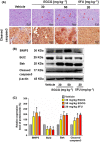
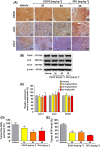
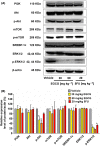
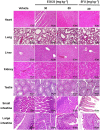
The de novo lipogenesis (DNL) pathway has been identified as a regulator of cancer progression and aggressiveness. Downregulation of key lipogenesis enzymes has been shown to activate apoptosis in cancerous cells. Epigallocatechin gallate (EGCG) inhibits cancer cell proliferation without causing cytotoxicity in healthy cells. The present study aimed to investigate the effects of EGCG on the promotion of apoptosis associated with the DNL pathway inhibition in cancer cells, both in vitro and in vivo. We observed that two colorectal cancer cell lines (HCT116 and HT-29) had a higher cytotoxic response to EGCG treatment than hepatocellular carcinoma cells, including HepG2 and HuH-7. EGCG treatment decreased cell viability and increased mitochondrial damage-triggered apoptosis in both HCT116 and HT-29 cancer cells. Additionally, we treated mice transplanted with HCT116 cells with 30 or 50 mg·kg-1 EGCG for 7 days to evaluate the apoptotic effects of EGCG treatment in a xenograft mouse model of cancer. We observed a decrease in intracellular fatty acid levels, which suggested that EGCG-induced apoptosis was associated with a decrease in fatty acid levels in cancer. Suppression of ATP synthesis by EGCG indicated that cell death induction in cancer cells could be mediated by shared components of the DNL and energy metabolism pathways. In addition, EGCG-induced apoptosis suppressed the expression of the phosphorylation protein kinase B and extracellular signal-regulated kinase 1/2 signaling proteins in tumors from xenografted mice. Cytotoxic effects in unaffected organs and tissues of the mouse xenograft model were absent upon EGCG treatment.
Keywords: PI3K/Akt/mTOR/SREBP-1c signaling; apoptosis; colorectal cancer; de novo lipogenesis; epigallocatechin gallate.
© 2022 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chandimali N, Sun H‐N, Kong L‐Z, Zhen X, Liu R, Kwon T, et al. Shikonin‐induced apoptosis of colon cancer cells is reduced by peroxiredoxin V expression. Anticancer Res. 2019;39:6115–23. - PubMed
-
- Zhu W, Li MC, Wang FR, Mackenzie GG, Oteiza PI. The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling. Biochem Pharmacol. 2020;175:113923. - PMC - PubMed
-
- Park J, Kim H‐D, Lee S‐H, Kwak C‐H, Chang Y‐C, Lee Y‐C, et al. Ascochlorin induces caspase‐independent necroptosis in LPS‐stimulated RAW 264.7 macrophages. J Ethnopharmacol. 2019;239:111898. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

