Associations of High-Sensitivity Troponin and Natriuretic Peptide Levels With Serious Adverse Events in SPRINT
- PMID: 35243872
- PMCID: PMC9075292
- DOI: 10.1161/JAHA.121.023314
Associations of High-Sensitivity Troponin and Natriuretic Peptide Levels With Serious Adverse Events in SPRINT
Abstract
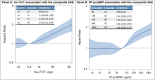
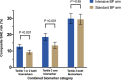
Background Assessing the risk of serious adverse events (SAEs) during hypertension treatment is important for understanding the benefit-harm trade-offs of lower blood pressure goals. It is unknown whether high-sensitivity cardiac troponin T (hs-cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) provide information about SAEs. Methods and Results In SPRINT (Systolic Blood Pressure Intervention Trial), hs-cTnT and NT-proBNP were measured at baseline in 8828 (94.3%) and 8836 (94.4%) participants, respectively. Multivariable Cox proportional hazards models were used to evaluate hs-cTnT and NT-proBNP associations with a composite of SPRINT's SAEs of interest: hypotension, syncope, bradycardia, acute kidney injury, electrolyte abnormalities, and injurious falls. Elevations in hs-cTnT and NT-proBNP were associated with increased composite SAE risk (hazard ratio [HR] per 2-fold higher hs-cTnT: 1.15; 95% CI, 1.06‒1.25; HR per 2-fold higher NT-proBNP: 1.09; 95% CI, 1.05‒1.14). Compared with both hs-cTnT and NT-proBNP in the lower tertiles, both biomarkers in the highest tertile was associated with increased composite SAE risk (HR, 1.56; 95% CI, 1.32‒1.84). Composite SAE risk was higher in the intensive-treatment group than in the standard-treatment group for participants with both biomarkers in the lower tertiles, but similar between treatment groups for participants with both biomarkers in the highest tertile (P for interaction=0.008). Conclusions Elevations in hs-cTnT and NT-proBNP individually and in combination are associated with higher composite SAE risk in SPRINT. The differential impact of blood pressure treatment on SAE risk across combined biomarker categories may have implications for identifying individuals with more favorable benefit-harm profiles for intensive blood pressure lowering.
Keywords: SPRINT; adverse events; brain natriuretic peptide; hypertension; troponin.
Figures

References
-
- GBD 2015 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Lond Engl. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8 - DOI - PMC - PubMed
-
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Cheryl DH, DePalma Sondra M, Samuel G, Jamerson KA, Jones DW, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. - PubMed
-
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- R01 DK098234/DK/NIDDK NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- K24 DK110427/DK/NIDDK NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- UL1 TR002548/TR/NCATS NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- P30 GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- R01 HL144112/HL/NHLBI NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

