Anticoagulants for people hospitalised with COVID-19
- PMID: 35244208
- PMCID: PMC8895460
- DOI: 10.1002/14651858.CD013739.pub2
Anticoagulants for people hospitalised with COVID-19
Abstract
Background: The primary manifestation of coronavirus disease 2019 (COVID-19) is respiratory insufficiency that can also be related to diffuse pulmonary microthrombosis and thromboembolic events, such as pulmonary embolism, deep vein thrombosis, or arterial thrombosis. People with COVID-19 who develop thromboembolism have a worse prognosis. Anticoagulants such as heparinoids (heparins or pentasaccharides), vitamin K antagonists and direct anticoagulants are used for the prevention and treatment of venous or arterial thromboembolism. Besides their anticoagulant properties, heparinoids have an additional anti-inflammatory potential. However, the benefit of anticoagulants for people with COVID-19 is still under debate.
Objectives: To assess the benefits and harms of anticoagulants versus active comparator, placebo or no intervention in people hospitalised with COVID-19.
Search methods: We searched the CENTRAL, MEDLINE, Embase, LILACS and IBECS databases, the Cochrane COVID-19 Study Register and medRxiv preprint database from their inception to 14 April 2021. We also checked the reference lists of any relevant systematic reviews identified, and contacted specialists in the field for additional references to trials.
Selection criteria: Eligible studies were randomised controlled trials (RCTs), quasi-RCTs, cluster-RCTs and cohort studies that compared prophylactic anticoagulants versus active comparator, placebo or no intervention for the management of people hospitalised with COVID-19. We excluded studies without a comparator group and with a retrospective design (all previously included studies) as we were able to include better study designs. Primary outcomes were all-cause mortality and necessity for additional respiratory support. Secondary outcomes were mortality related to COVID-19, deep vein thrombosis, pulmonary embolism, major bleeding, adverse events, length of hospital stay and quality of life.
Data collection and analysis: We used standard Cochrane methodological procedures. We used Cochrane RoB 1 to assess the risk of bias for RCTs, ROBINS-I to assess risk of bias for non-randomised studies (NRS) and GRADE to assess the certainty of evidence. We meta-analysed data when appropriate.
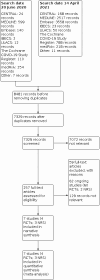
Main results: We included seven studies (16,185 participants) with participants hospitalised with COVID-19, in either intensive care units, hospital wards or emergency departments. Studies were from Brazil (2), Iran (1), Italy (1), and the USA (1), and two involved more than country. The mean age of participants was 55 to 68 years and the follow-up period ranged from 15 to 90 days. The studies assessed the effects of heparinoids, direct anticoagulants or vitamin K antagonists, and reported sparse data or did not report some of our outcomes of interest: necessity for additional respiratory support, mortality related to COVID-19, and quality of life. Higher-dose versus lower-dose anticoagulants (4 RCTs, 4647 participants) Higher-dose anticoagulants result in little or no difference in all-cause mortality (risk ratio (RR) 1.03, 95% CI 0.92 to 1.16, 4489 participants; 4 RCTs) and increase minor bleeding (RR 3.28, 95% CI 1.75 to 6.14, 1196 participants; 3 RCTs) compared to lower-dose anticoagulants up to 30 days (high-certainty evidence). Higher-dose anticoagulants probably reduce pulmonary embolism (RR 0.46, 95% CI 0.31 to 0.70, 4360 participants; 4 RCTs), and slightly increase major bleeding (RR 1.78, 95% CI 1.13 to 2.80, 4400 participants; 4 RCTs) compared to lower-dose anticoagulants up to 30 days (moderate-certainty evidence). Higher-dose anticoagulants may result in little or no difference in deep vein thrombosis (RR 1.08, 95% CI 0.57 to 2.03, 3422 participants; 4 RCTs), stroke (RR 0.91, 95% CI 0.40 to 2.03, 4349 participants; 3 RCTs), major adverse limb events (RR 0.33, 95% CI 0.01 to 7.99, 1176 participants; 2 RCTs), myocardial infarction (RR 0.86, 95% CI 0.48 to 1.55, 4349 participants; 3 RCTs), atrial fibrillation (RR 0.35, 95% CI 0.07 to 1.70, 562 participants; 1 study), or thrombocytopenia (RR 0.94, 95% CI 0.71 to 1.24, 2789 participants; 2 RCTs) compared to lower-dose anticoagulants up to 30 days (low-certainty evidence). It is unclear whether higher-dose anticoagulants have any effect on necessity for additional respiratory support, mortality related to COVID-19, and quality of life (very low-certainty evidence or no data). Anticoagulants versus no treatment (3 prospective NRS, 11,538 participants) Anticoagulants may reduce all-cause mortality but the evidence is very uncertain due to two study results being at critical and serious risk of bias (RR 0.64, 95% CI 0.55 to 0.74, 8395 participants; 3 NRS; very low-certainty evidence). It is uncertain if anticoagulants have any effect on necessity for additional respiratory support, mortality related to COVID-19, deep vein thrombosis, pulmonary embolism, major bleeding, stroke, myocardial infarction and quality of life (very low-certainty evidence or no data). Ongoing studies We found 62 ongoing studies in hospital settings (60 RCTs, 35,470 participants; 2 prospective NRS, 120 participants) in 20 different countries. Thirty-five ongoing studies plan to report mortality and 26 plan to report necessity for additional respiratory support. We expect 58 studies to be completed in December 2021, and four in July 2022. From 60 RCTs, 28 are comparing different doses of anticoagulants, 24 are comparing anticoagulants versus no anticoagulants, seven are comparing different types of anticoagulants, and one did not report detail of the comparator group.
Authors' conclusions: When compared to a lower-dose regimen, higher-dose anticoagulants result in little to no difference in all-cause mortality and increase minor bleeding in people hospitalised with COVID-19 up to 30 days. Higher-dose anticoagulants possibly reduce pulmonary embolism, slightly increase major bleeding, may result in little to no difference in hospitalisation time, and may result in little to no difference in deep vein thrombosis, stroke, major adverse limb events, myocardial infarction, atrial fibrillation, or thrombocytopenia. Compared with no treatment, anticoagulants may reduce all-cause mortality but the evidence comes from non-randomised studies and is very uncertain. It is unclear whether anticoagulants have any effect on the remaining outcomes compared to no anticoagulants (very low-certainty evidence or no data). Although we are very confident that new RCTs will not change the effects of different doses of anticoagulants on mortality and minor bleeding, high-quality RCTs are still needed, mainly for the other primary outcome (necessity for additional respiratory support), the comparison with no anticoagulation, when comparing the types of anticoagulants and giving anticoagulants for a prolonged period of time.
Trial registration: ClinicalTrials.gov NCT04394377 NCT04486508 NCT04354155 NCT04359212 NCT04368377 NCT04393805 NCT04483830 NCT04504032 NCT04736901 NCT04828772 NCT04528888 NCT04360824 NCT04401293 NCT04445935 NCT04389840 NCT04487990 NCT04408235 NCT04344756 NCT04345848 NCT04352400 NCT04366960 NCT04367831 NCT04373707 NCT04397510 NCT04406389 NCT04409834 NCT04416048 NCT04420299 NCT04485429 NCT04530578 NCT04584580 NCT04604327 NCT04362085.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
RLGF: none known VTC: none known JDST: none known PIFP: none known LLA: none known CM: none known BT: none known VFMT: none known ANA: none known LCUN: none known
Figures
















































Update of
-
Prophylactic anticoagulants for people hospitalised with COVID-19.Cochrane Database Syst Rev. 2020 Oct 2;10(10):CD013739. doi: 10.1002/14651858.CD013739. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Mar 4;3:CD013739. doi: 10.1002/14651858.CD013739.pub2. PMID: 33502773 Free PMC article. Updated.
Comment in
-
Benefits and Harms of Anticoagulants in People Hospitalized With COVID-19.Am Fam Physician. 2022 Nov;106(5):501-502. Am Fam Physician. 2022. PMID: 36379495 No abstract available.
References
References to studies included in this review
Albani 2020 {published data only}
-
- Albani F, Sepe L, Fusina F, Prezioso C, Baronio M, Caminiti F, et al.Thromboprophylaxis with enoxaparin is associated with a lower death rate in patients hospitalized with SARS-CoV-2 infection. A cohort study. EClinicalMedicine 2020;27:100562. [DOI: 10.1016/j.eclinm.2020.100562] - DOI - PMC - PubMed
Lemos 2020 {published data only (unpublished sought but not used)}
-
- RBR-949z6v.Full versus prophylactic heparinization for the treatment of severe forms of SARS-COVID-19: clinical, randomized, open and controlled study - HeSAcovid trial. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=RBR-949z6v (first received 23 February 2021).
-
- RBR-949z6v.RBR-949z6v Full versus prophylactic anticoagulation for the treatment of severe forms of COVID-19. ensaiosclinicos.gov.br/rg/RBR-949z6v/ (first received 6 April 2020).
Lopes 2021 {published data only}
-
- Bavry AA, Bhatt DL.AnticoagulatIon coronavirus - ACTION. acc.org/Latest-in-Cardiology/Clinical-Trials/2021/05/14/03/11/ACTION#.YK... (assessed 16 May 2021).
-
- Lopes RD, Silva PG, Furtado RH, Macedo AVS, Ramacciotti E, Damini LP, et al.Randomized clinical trial to evaluate a routine full anticoagulation strategy in patients with coronavirus Infection (SARS-CoV2) admitted to hospital: rationale and design of the ACTION (AntiCoagulaTlon cOroNavirus)-Coalition IV trial. American Heart Journal 2021;238:1-11. [DOI: 10.1016/j.ahj.2021.04.005] - DOI - PMC - PubMed
-
- Lopes RD, Silva PG, Furtado RH, Macedo AV, Bronhara B, Damiani LP, et al.Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet 2021;397(10291):2253-63. [DOI: 10.1016/S0140-6736(21)01203-4] - DOI - PMC - PubMed
-
- Lopes RD.ACC.21 Presentation Slides | ACTION. acc.org/education-and-meetings/image-and-slide-gallery/media-detail?id=D... (assessed 16 May 2021).
-
- Lopes RD.Details about your trial data [ACTION trial] [personal communication]. Email to: RL Flumignan 22 May 2021.
Rentsch 2020 {published data only}
-
- Rentsch CT, Beckman JA, Tomlinson L, Gellad WF, Alcorn C, Kidwai-Khan F, et al.Early initiation of prophylactic anticoagulation for prevention of COVID-19 mortality: a nationwide cohort study of hospitalized patients in the United States. medRxiv [Preprint}. [DOI: 10.1101/2020.12.09.20246579] - DOI - PMC - PubMed
Sadeghipour 2021 {published data only (unpublished sought but not used)}
-
- Bikdeli B, Talasaz AH, Rashidi F, Bakhshandeh H, Rafiee F, Matin S, et al.Intermediate vs standard-dose prophylactic anticoagulation in patients with COVID-19 admitted to ICU: ninety-day results from the INSPIRATION Trial. Thrombosis and Haemostasis 2022;122(1):131-41. [DOI: 10.1055/a-1485-2372] - DOI - PubMed
-
- Bikdeli B, Talasaz AH, Rashidi F, Sharif-Kashani B, Farrokhpour M, Bakhshandeh H, et al.Intermediate versus standard-dose prophylactic anticoagulation and statin therapy versus placebo in critically-ill patients with COVID-19: rationale and design of the INSPIRATION/INSPIRATION-S studies. Thrombosis Research 2020;196:382‐94. [DOI: 10.1016/j.thromres.2020.09.027] - DOI - PMC - PubMed
-
- Bikdeli B.Details about your trial data [INSPIRATION trial] [personal communication]. Email to: RLG Flumignan 4 June 2021.
-
- NCT04486508.Intermediate-dose vs standard prophylactic anticoagulation and statin vs placebo in ICU patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04486508 (first received 22 July 2020).
Santoro 2020 {published data only (unpublished sought but not used)}
-
- Núñez-Gil IJ, Estrada V, Fernández-Pérez C, Feltes G, Vedia O, Vergara-Uzcategui CE.Health outcome predictive evaluation for COVID 19 international registry (HOPE COVID-19), rationale and design. Contemporary Clinical Trials Communication 2020;20:100654. [DOI: 10.1016/j.conctc.2020.100654] - DOI - PMC - PubMed
-
- NCT04334291.International COVID19 clinical evaluation registry, (HOPE COVID 19). clinicaltrials.gov/ct2/show/NCT04334291 (first received 1 April 2020).
-
- NCT04334291.International COVID-19 clinical evaluation registry 2: HOPE 2. hopeprojectmd.com/en/ (first received 1 April 2020).
-
- Rivera-Caravaca JM, Núñez-Gil IJ, Vivas D, Viana-Llamas MC, Uribarri A, Becerra-Muñoz VM, et al.Clinical profile and prognosis in patients on oral anticoagulation before admission for COVID-19. European Journal of Clinical Investigation 2021;51(1):e13436. [DOI: 10.1111/eci.13436] - DOI - PMC - PubMed
-
- Santoro F, Nunez I, Pepe M, Estrada V, Brunetti ND.A289: Anticoagulation therapy in patients with COVID-19. Results from a multi-center international prospective registry (HOPE-COVID19). Giornale Italiano di Cardiologia 2020;21(12 Suppl 2):e90.
Zarychanski 2021 {published data only (unpublished sought but not used)}
-
- EUCTR2015-002340-14-NL.Randomized, embedded, multifactorial, adaptive platform trial for community-acquired pneumonia (REMAP-CAP). (COVID-19) - REMAP-CAP. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2015-002340-14-NL (first received 16 September 2015).
-
- EUCTR2020-004285-19.A multicenter, adaptive, randomized controlled platform trial of the safety and efficacy of antithrombotic strategies in hospitalized adults with COVID-19. clinicaltrialsregister.eu/ctr-search/trial/2020-004285-19/IT (first received 23 November 2020).
-
- Houston BL, Lawler PR, Goligher EC, Farkouh ME, Bradbury C, Carrier M, et al.Anti-thrombotic therapy to ameliorate complications of COVID-19 (ATTACC): study design and methodology for an international, adaptive Bayesian randomized controlled trial. Clinical Trials 2020;17(5):491‐500. [DOI: 10.1177/1740774520943846] - DOI - PubMed
-
- Lawler P, Goligher E, Berger J, Neal M, McVerry B, Nicolau J, et al.Therapeutic anticoagulation in non-critically Ill patients with COVID-19. medRxiv [Preprint]. [DOI: 10.1101/2021.05.13.21256846] - DOI
References to studies excluded from this review
Al‐Samkari 2020 {published data only}
Artifoni 2020 {published data only}
-
- Artifoni M, Danic G, Gautier G, Gicquel P, Boutoille D, Raffi F, et al.Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. Journal of Thrombosis and Thrombolysis 2020;50(1):211-6. [PMID: ] - PMC - PubMed
Ayerbe 2020 {published data only}
ChiCTR2000034796 {published data only}
-
- ChiCTR2000034796.The efficacy and safety of heparin in the treatment of novel coronavirus pneumonia (COVID-19): a retrospective single center clinical trial. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ChiCTR2000034796 (first received 18 January 2020).
CTRI/2021/01/030373 {published data only}
-
- CTRI/2021/01/030373.ETHIC trial: early LMWH in symptomatic COVID-19 positive patients. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=CTRI/2021/01/030373 (first received 11 January 2021).
D'Ardes 2021 {published data only}
DCTC 2021 {published data only}
-
- Dutch COVID & Thrombosis Coalition.Early effects of unfractionated heparin on clinical and radiological signs and D-dimer levels in patients with COVID-19 associated pulmonary embolism: an observational cohort study. Thrombosis Research 2021;200:130-2. [DOI: 10.1016/j.thromres.2021.01.023] - DOI - PMC - PubMed
Di Castelnuovo 2021 {published data only}
EUCTR2020‐001748‐24‐SE {published data only}
-
- EUCTR2020-001748-24-SE.A multi-center, randomized, open-label study in patients with COVID-19 and respiratory distress not requiring mechanical ventilation, to compare standard-of-care with anakinra and tocilizumab treatment. The immunomodulation-CoV assessment (ImmCoVA) study. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2020-001748-24-SE (first received 20 April 2020).
EudraCT2020‐001823‐15 {published data only}
-
- EudraCT2020-001823-15.Evaluation of the concentration-effect relationship of enoxaparin for thromboembolic prevention in COVID-19 resuscitation patients. COV-ENOX study. clinicaltrialsregister.eu/ctr-search/trial/2020-001823-15/FR (first received 15 April 2020).
Falcone 2020 {published data only}
-
- Falcone M, Tiseo G, Barbieri G, Galfo V, Russo A, Virdis A, et al.Role of low-molecular-weight heparin in hospitalized patients with severe acute respiratory syndrome coronavirus 2 pneumonia: a prospective observational study. Open Forum Infectious Diseases 2020;7(12):ofaa563. [DOI: 10.1093/ofid/ofaa563] - DOI - PMC - PubMed
Frohlich 2021 {published data only}
-
- Frohlich GM, Jeschke E, Eichler U, Thiele H, Alhariri L, Reinthaler M, et al.Impact of oral anticoagulation on clinical outcomes of COVID-19: a nationwide cohort study of hospitalized patients in Germany. Clinical Research in Cardiology 2021;110(7):1041-50. [PMID: 10.1007/s00392-020-01783-x] - DOI - PMC - PubMed
Helms 2020 {published data only}
Helms 2021 {published data only}
Ho 2021 {published data only}
Huang 2020 {published data only}
Ionescu 2020 {published data only}
Jiménez‐Soto 2021 {published data only}
Jonmarker 2020 {published data only}
Khider 2020 {published data only}
Kodama 2020 {published data only}
-
- Kodama R, Kalsi A, Singh A, Vishnuvardhan N, Nimkar N, Paul S, et al.Outcomes in COVID-19 patients on treatment dose anti-coagulation compared to prophylactic dose anti-coagulation. Blood 2020;136 Suppl 1:40-1. [DOI: 10.1182/blood-2020-142552] - DOI
Kow 2020 {published data only}
Kukin 2020 {published data only}
-
- Kukin A, Gale SE, Reed BN, Devabhakthuni S, Watson K, Noel Z.Effect of video-assisted counseling versus traditional counseling on patient comprehension of prescribed direct oral anticoagulants. Journal of the American College of Clinical Pharmacy 2020;3(8):1538‐9. [DOI: 10.1002/jac5.1351] - DOI
Liu 2020 {published data only}
-
- Liu X, Zhang X, Xiao Y, Gao T, Wang G, Wang Z, et al.Heparin-induced thrombocytopenia is associated with a high risk of mortality in critical COVID-19 patients receiving heparin-involved treatment. medRxiv [Preprint]. [DOI: 10.1101/2020.04.23.20076851] - DOI
Mareev 2020 {published data only}
-
- Mareev VY, Orlova YA, Pavlikova EP, Akopyan ZA, Matskeplishvili ST, Plisyk AG, et al.Proactive anti-inflammatory and anticoagulant therapy in the treatment of advanced stages of novel coronavirus infection (COVID-19). Case series and study design: coLchicine versus ruxolitinib and secukinumab in open prospective randomIzed trial (COLORIT). Kardiologiia 2020;60(9):4‐21. [DOI: 10.18087/cardio.2020.9.n1338] - DOI - PubMed
-
- NCT04403243.Colchicine versus ruxolitinib and secukinumab in open prospective randomized trial (COLORIT). clinicaltrials.gov/ct2/show/NCT04403243 (first received 20 May 2020).
Martinelli 2020 {published data only}
-
- Martinelli I, Ciavarella A, Abbattista M, Aliberti S, De Zan V, Folli C, et al.Increased doses of low-molecular-weight heparin in hospitalized patients with COVID-19. Research and Practice in Thrombosis and Haemostasis 2020;4 Suppl 2:15-6. [DOI: 10.1002/rth2.12413] - DOI
Maurer 2020 {published data only}
-
- Maurer L, Luckhurst C, Hamidi A, Newman K, Barra M, El Hechi M, et al.A low dose heparin protocol is associated with improved duration of arterial line patency in critically ill COVID-19 patients. Research and Practice in Thrombosis and Haemostasis 2020;4 Suppl 2:8-9. [DOI: 10.1002/rth2.12413] - DOI - PMC - PubMed
-
- Maurer LR, Luckhurst CM, Hamidi A, Newman KA, Barra ME, El Hechi M, et al.A low dose heparinized saline protocol is associated with improved duration of arterial line patency in critically ill COVID-19 patients. Journal of Critical Care 2020;60:253-9. [DOI: 10.1016/j.jcrc.2020.08.025] - DOI - PMC - PubMed
NCT04354155 {published data only}
-
- NCT04354155.COVID-19 anticoagulation in children - thromboprophlaxis (COVAC-TP) trial. clinicaltrials.gov/ct2/show/NCT04354155 (first received 21 April 2020).
NCT04359212 {published data only}
-
- NCT04359212.Increased risk of venous thromboembolism and higher hypercoagulable state in patients recovered in intensive care unit and in medical ward for coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04359212 (first received 21 April 2020).
NCT04365309 {published data only}
-
- NCT04365309.Protective effect of aspirin on COVID-19 patients (PEAC). clinicaltrials.gov/ct2/show/NCT04365309 (first received 28 April 2020).
NCT04368377 {published data only}
-
- NCT04368377.Platelet inhibition with GP IIb/IIIa inhibitor in critically ill patients with coronavirus disease 2019 (COVID-19). A compassionate use protocol. clinicaltrials.gov/ct2/show/NCT04368377 (first received 29 April 2020).
NCT04393805 {published data only}
-
- NCT04393805.Heparins for thromboprophylaxis in COVID-19 patients: HETHICO study in Veneto. clinicaltrials.gov/ct2/show/NCT04393805 (first received 16 May 2020).
NCT04427098 {published data only}
-
- 2020-001308-40.Intermediate dose enoxaparin in hospitalized patients with moderate-severe COVID-19: a pilot phase II single-arm study, INHIXACOVID19. clinicaltrialsregister.eu/ctr-search/trial/2020-001308-40/IT (first received 24 June 2020). - PMC - PubMed
-
- NCT04427098.Intermediate dose enoxaparin in hospitalized patients with moderate-severe COVID19: a pilot phase II single-arm study, INHIXACOVID19. clinicaltrials.gov/ct2/show/study/NCT04427098 (first received 11 June 2020).
NCT04483830 {published data only}
-
- NCT04483830.Suloexide in the treatment of early stages of COVID-19 (SulES-COVID). clinicaltrials.gov/ct2/show/NCT04483830 (first received 22 July 2020) (first received 22 July 2020).
NCT04492254 {published data only}
-
- NCT04492254.Early prophylactic low-molecular-weight heparin (LMWH) in symptomatic COVID-19 positive patients (ETHIC). clinicaltrials.gov/ct2/show/NCT04492254 (first received 22 July 2020).
NCT04504032 {published data only}
-
- EUCTR2020-005395-35.A randomized, controlled, Phase 2b study to evaluate safety and efficacy of rivaroxaban (Xarelto®) for high risk people with mild COVID-19. clinicaltrialsregister.eu/ctr-search/trial/2020-005395-35/GB (first received 26 November 2020).
-
- NCT04504032.A trial to evaluate safety and efficacy of rivaroxaban (COVID-19). clinicaltrials.gov/ct2/show/NCT04504032 (first received 5 August 2020).
NCT04516941 {published data only}
-
- NCT04516941.Corona virus edoxaban colchicine (CONVINCE) COVID-19 (CONVINCE). clinicaltrials.gov/ct2/show/NCT04516941 (first received 5 August 2020).
NCT04662684 {published data only}
-
- NCT04662684.Medically Ill hospitalized patients for COVID-19 thrombosis extended prophylaxis with rivaroxaban therapy: the MICHELLE Trial. clinicaltrials.gov/ct2/show/NCT04662684 (first received 8 December 2020).
NCT04673214 {published data only}
-
- NCT04673214.Evaluation of prognostic modification in COVID-19 patients in early intervention treatment. clinicaltrials.gov/ct2/show/NCT04673214 (first received 16 December 2020).
NCT04715295 {published data only}
-
- NCT04715295.Safety and efficacy of doxycycline and rivaroxaban in COVID-19 (DOXYCOV). clinicaltrials.gov/ct2/show/NCT04715295 (first received 2 October 2020).
NCT04736901 {published data only}
-
- NCT04736901.Effect of prophylactic and therapeutic anticoagulants in Egyptian patients with COVID-19. clinicaltrials.gov/ct2/show/results/NCT04736901?view=results (first received 3 February 2021).
NCT04757857 {published data only}
-
- NCT04757857.COVID-19 antithrombotic rivaroxaban evaluation (CARE). clinicaltrials.gov/ct2/show/NCT04757857 (first received 8 February 2021).
NCT04828772 {published data only}
-
- NCT04828772.A study of anticoagulation treatment patterns and outcomes of participants hospitalized with coronavirus disease 2019 (COVID-19) in Japan. clinicaltrials.gov/ct2/show/NCT04828772 (first received 25 March 2021).
Paranjpe 2020 {published data only}
Piagnerelli 2020 {published data only}
Poulakou 2021 {published data only}
-
- Poulakou G, Dimakakos E, Kollias A, Kyriakoulis KG, Rapti V, Trontzas I, et al.Beneficial effects of intermediate dosage of anticoagulation treatment on the prognosis of hospitalized COVID-19 patients: the ETHRA Study. In Vivo (Athens, Greece) 2021;35(1):653-61. [DOI: 10.21873/invivo.12305] - DOI - PMC - PubMed
Qin 2021 {published data only}
Rosovsky 2020 {published data only}
Russo 2020 {published data only}
-
- Russo V, Di Maio M, Attena E, Silverio A, Scudiero F, Celentani D, et al.Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: a multicenter observational study. Pharmacological Research 2020;159:104965. [DOI: 10.1016/j.phrs.2020.104965] - DOI - PMC - PubMed
Secco 2020 {published data only}
Shi 2020 {published data only}
Sivaloganathan 2020 {published data only}
Smith 2020 {published data only}
-
- Smith SR, Waite A, Johnston BW, Welters ID.P520 Survey of current practice in management of anticoagulation in adult critically ill patients with COVID-19 in the northwest of England. Critical Care 2020;24 Suppl 2:P520. [PMID: 10.1186/s13054-020-03187-9] - DOI
Stessel 2020 {published data only}
-
- NCT04394000.Impact of implementation of an intensified thromboprofylaxis protocol in critically ill ICU patients with COVID-19: a longitudinal controlled before-after study. clinicaltrials.gov/ct2/show/record/NCT04394000 (first received 19 May 2020).
-
- Stessel B, Vanvuchelen C, Bruckers L, Geebelen L, Callebaut I, Vandenbrande J, et al.Impact of implementation of an individualised thromboprophylaxis protocol in critically ill ICU patients with COVID-19: a longitudinal controlled before-after study. Thrombosis Research 2020;194:209-15. [DOI: 10.1016/j.thromres.2020.07.038] - DOI - PMC - PubMed
Tacquard 2021 {published data only}
-
- NCT04405869.Thromboembolic events in severe COVID-19 patients (COVICLOT). clinicaltrials.gov/ct2/show/NCT04405869 (first received 25 May 2020).
Tamargo 2021 {published data only}
Tang 2020 {published data only}
Trinh 2020 {published data only}
-
- Trinh M, Chang DR, Govindarajulu US, Kane E, Fuster V, Kohli-Seth R, et al.Therapeutic anticoagulation is associated with decreased mortality in mechanically ventilated COVID-19 patients. medRxiv [Preprint]. [DOI: 10.1101/2020.05.30.20117929] - DOI
Zhang 2020 {published data only}
-
- Zhang Y, Cao W, Xiao M, Li YJ, Yang Y, Zhao J, et al.Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue za Zhi 2020;41(0):E006. [PMID: ] - PubMed
References to ongoing studies
ACTRN12620000517976 {published data only}
-
- ACTRN12620000517976.A randomised controlled trial of nebulised heparin in critically ill mechanically ventilated patients with COVID-19 to assess the effect on the duration of mechanical ventilation. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000517976 (first received 27 April 2020).
-
- ACTRN12620000517976.Can nebulised heparin reduce time to extubation in SARS CoV 2. The CHARTER study protocol. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12620000517976 (first received 27 April 2020).
-
- Dixon B, Smith RJ, Artigas A, Laffey J, McNicholas B, Schmidt E, et al.Can nebulised heparin reduce time to extubation in SARS CoV 2. The CHARTER study protocol. medRxiv [Preprint]. [DOI: 10.1101/2020.04.28.20082552] - DOI
Busani 2020 {published data only}
-
- Busani S, Tosi M, Mighali P, Vandelli P, D'Amico R, Marietta M, et al.Multi-centre, three arm, randomized controlled trial on the use of methylprednisolone and unfractionated heparin in critically ill ventilated patients with pneumonia from SARS-CoV-2 infection: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21:724. [DOI: 10.1186/s13063-020-04645-z] - DOI - PMC - PubMed
-
- EUCTR2020-001921-30.Steroids and unfractionated heparin in critically-ill patients with pneumonia from COVID-19 infection. A multicenter, interventional, randomized, three arms study design. clinicaltrialsregister.eu/ctr-search/trial/2020-001921-30/IT (first received 26 June 2020).
-
- NCT04528888.Steroids and unfractionated heparin in critically-ill patients with pneumonia from COVID-19 infection. A multicenter, interventional, randomized, three arms study design. clinicaltrials.gov/ct2/show/NCT04528888 (first received 21 August 2020).
Chambers 2020 {published data only}
-
- Chambers I, Dayal S, Eyck PT, Eustes A, Sutamtewagul G, Rosenstein L, et al.COVID-19-associated coagulopathy: safety and efficacy of prophylactic anticoagulation therapy in hospitalized adults with COVID-19. Blood 2020;136 Suppl 1:11. [10.1182/blood-2020-141951]
-
- NCT04360824.COVID-19-associated coagulopathy: safety and efficacy of prophylactic anticoagulation therapy in hospitalized adults with COVID-19. clinicaltrials.gov/ct2/show/NCT04360824 (first received 24 April 2020).
ChiCTR2000030700 {published data only}
-
- ChiCTR2000030700.Study for the efficacy and safety of prolongin (enoxaparin sodium injection) in treatment of novel coronavirus pneumonia (COVID-19) adult common patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000030700 (first received 10 March 2020).
-
- ChiCTR2000030700.Study for the efficacy and safety of prolongin (enoxaparin sodium injection) in treatment of novel coronavirus pneumonia (COVID-19) adult common patients. www.chictr.org.cn/showprojen.aspx?proj=50786 (first received 10 March 2020).
ChiCTR2000030701 {published data only}
-
- ChiCTR2000030701.A randomized, parallel controlled open-label trial for the efficacy and safety of prolongin (enoxaparin sodium injection) in the treatment of adult patients with novel coronavirus pneumonia (COVID-19). chictr.org.cn/showprojen.aspx?proj=50795 (first received 10 March 2020).
-
- ChiCTR2000030701.A randomized, parallel controlled open-label trial to evaluate the efficacy and safety of Prolongin (enoxaparin sodium injection) in adult hospitalized patients with novel coronavirus pneumonia (COVID-19). ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ChiCTR2000030701 (first received 10 March 2020).
ChiCTR2000030946 {published data only}
-
- ChiCTR2000030946.Effects of different VTE prevention methods on the prognosis of hospitalized patients with novel coronavirus pneumonia (COVID-19). chictr.org.cn/showprojen.aspx?proj=51265 (first received 19 March 2020).
CTRI/2020/06/026220 {published data only}
-
- CTRI/2020/06/026220.An open label, randomized, multicenter, controlled clinical study to evaluate the efficacy and safety of nafamostat mesilate in the treatment of moderate COVID-19 disease. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=CTRI/2020/06/026220 (first received 17 July 2020).
CTRI/2020/08/027033 {published data only}
-
- CTRI/2020/08/027033.SARS-COV-2 and COVID-19 - a randomized controlled trail (unblinded). ictrptest.azurewebsites.net/Trial2.aspx?TrialID=CTRI/2020/08/027033 (first received 07 August 2020).
CTRI/2020/11/029175 {published data only}
-
- CTRI/2020/11/029175.Role of heparin inhalation in reducing the duration the patient is breathing with the help of a ventilator. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=CTRI/2020/11/029175 (first received 17 November 2020).
CTRI/2020/11/029345 {published data only}
-
- CTRI/2020/11/029345.To determine efficacy, safety and optimal dosing of anticoagulant strategies to prevent adverse outcomes in hospitalized COVID-19 patients. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=CTRI/2020/11/029345 (first received 25 November 2020).
EUCTR2020‐001302‐30‐AT {published data only}
-
- EUCTR2020-001302-30-AT.A multicenter, randomized, active controlled, open label, platform trial on the efficacy and safety of experimental therapeutics for patients with a lung disease caused by coronavirus infection ACOVACT (Austrian CoronaVirus Adaptive Clinical Trial). ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2020-001302-30-AT (first received 09 April 2020).
EUCTR2020‐001708‐41‐IT {published data only}
-
- EUCTR2020‐001708‐41‐IT.Enoxaparin for thromboprophylaxis in hospitalized COVID-19 patients: comparison of 40 mg o.d. versus 40 mg b.i.d. A randomized clinical trial. clinicaltrialsregister.eu/ctr-search/trial/2020-001708-41/IT (first received 24 June 2020).
EUCTR2020‐001709‐21‐FR {published data only}
-
- EUCTR2020‐001709‐21‐FR.Effectiveness of low molecular weight heparin at increased doses prophylaxis weight-adjusted, compared with lower doses prophylaxis (intermediate or standard), on the onset of venous thromboembolism in coronavirus disease 2019 (COVID-19) hospitalized patients: the randomized multicentric controlled open-label trial COVI-DOSE. clinicaltrialsregister.eu/ctr-search/trial/2020-001709-21/FR (first received 24 March 2020).
-
- EUCTR2020‐001709‐21‐FR.Low-molecular-weight heparin to prevent venous thromboembolism in COVID-19 patients: a randomized controlled trial of different doses. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2020-001709-21-FR (first received 29 April 2020).
EUCTR2020‐001891‐14‐ES {published data only}
-
- EUCTR2020-001891-14-ES.Impact of the use of low molecular weight heparins (LMWH), at prophylactic versus intermediate doses, on SARS-CoV2 infection (COVID-19). ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2020-001891-14-ES (first received 05 May 2020).
EUCTR2020‐002234‐32‐IT {published data only}
-
- EUCTR2020-002234-32-IT.Efficacy and safety of edoxaban and or colchicine for patients with SARS-CoV-2 infection managed in the out of hospital setting (COVID 19) - CorONa Virus edoxabaN ColchicinE (CONVINCE). ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2020-002234-32-IT (first received 4 January 2021).
EUCTR2020‐002504‐39‐DE {published data only}
-
- EUCTR2020-002504-39-DE.Hamburg edoxaban for anticoagulation in COVID-19 study - HERO-19. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2020-002504-39-DE (first received 14 August 2020).
EUCTR2020‐003349‐12‐IE {published data only}
-
- EUCTR2020-003349-12-IE.This is a proof of principle / feasibility study aiming to evaluate the effect of nebulised unfractionated heparin on procoagulant markers related to acute respiratory distress syndrome in patients invasively ventilated for COVID 19 lung disease - Charter Trial. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2020-003349-12-IE (first received 14 August 2020).
Goldin 2020 {published data only}
-
- Goldin M, Giannis D, Diab W, Wang J, Khanijo S, Sharifova G, et al.Treatment-dose LMWH versus prophylactic/intermediate dose heparins in high risk COVID-19 inpatients: rationale and design of the HEP-COVID Trial. Thrombosis and Haemostasis 2021;121(12):1684-95. [DOI: 10.1055/a-1475-2351] - DOI - PubMed
-
- NCT04401293.Systemic anticoagulation with full dose low molecular weight heparin (LMWH) vs. prophylactic or intermediate dose LMWH in high risk COVID-19 patients (HEP-COVID Trial). clinicaltrials.gov/ct2/show/NCT04401293 (first received 26 May 2020).
IRCT20200515047456N1 {published data only}
-
- IRCT20200515047456N1.The role of anticoagulant and thrombolitic in treatment of COVID patients. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=IRCT20200515047456N1 (first received 28 June 2020).
ISRCTN14212905 {published data only}
-
- ISRCTN14212905.Understanding how COVID-19 leads to respiratory failure in COVID-19 positive patients. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN14212905 (first received 03 July 2020).
Kharma 2020 {published data only}
-
- Kharma N, Roehrig S, Shible AA, Elshafei MS, Osman D, Elsaid IM, et al.Anticoagulation in critically ill patients on mechanical ventilation suffering from COVID-19 disease, The ANTI-CO trial: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):769. [PMID: ] - PMC - PubMed
-
- NCT04445935.Anticoagulation in patients suffering from COVID-19 disease the ANTI-CO Trial. clinicaltrials.gov/ct2/show/NCT04445935 (first received 23 June 2020).
Lasky 2021 {published data only}
-
- Lasky JA, Fuloria J, Morrison ME, Lanier R, Naderer O, Brundage T, et al.Design and rationale of a randomized, double-blind, placebo-controlled, phase 2/3 study evaluating dociparstat in acute lung injury associated with severe COVID-19. Advances in Therapy 2021;38(1):782‐91. [DOI: 10.1007/s12325-020-01539-z] - DOI - PMC - PubMed
-
- NCT04389840.Dociparstat for the treatment of severe COVID-19 in adults at high risk of respiratory failure. clinicaltrials.gov/ct2/show/NCT04389840 (first received 13 May 2020).
Lins 2020 {published data only}
-
- Lins PR, Albuquerque CC, Assis CF, Rodrigues BC, e Siqueira Campos BP, Oliveira Valle E, et al.CoV-Hep study: heparin in standard anticoagulation based on citrate for continuous veno-venous hemodialysis in patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials 2020;21(1):920. [DOI: 10.1186/s13063-020-04814-0] - DOI - PMC - PubMed
-
- NCT04487990.CoV-Hep study: comparative study between different anti-coagulation strategies in continuous hemodialysis in COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04487990 (first received 23 July 2020).
Marietta 2020 {published data only}
-
- EUCTR2020-001972-13-IT.High versus low LMWH dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy COVID-19 HD. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=NCT04408235 (first received 26 May 2020).
-
- Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D'Amico R.Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD). Blood Transfusion 2020;18 Suppl 4:S490. [DOI: 10.2450/2020.S4] - DOI - PMC - PubMed
-
- Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D'Amico R.Randomised controlled trial comparing efficacy and safety of high versus low molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials 2020;21(1):574. [PMID: ] - PMC - PubMed
-
- NCT04408235.High versus low LMWH dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy (COVID-19 HD). clinicaltrials.gov/ct2/show/NCT04408235 (first received 29 May 2020).
NCT04333407 {published data only}
-
- NCT04333407.Preventing cardiac complication of COVID-19 disease with early acute coronary syndrome therapy: a randomised controlled trial. clinicaltrials.gov/ct2/show/NCT04333407 (first received 3 April 2020).
NCT04344756 {published data only}
-
- NCT04344756.Trial evaluating efficacy and safety of anticoagulation in patients with COVID-19 infection, nested in the Corimmuno-19 cohort (CORIMMUNO-COAG). clinicaltrials.gov/ct2/show/study/NCT04344756 (first received 14 April 2020).
NCT04345848 {published data only}
-
- NCT04345848.Preventing COVID-19-associated thrombosis, coagulopathy and mortality with low- and high-dose anticoagulation: a randomized, open-label clinical trial. clinicaltrials.gov/ct2/show/NCT04345848 (first received 15 April 2020).
NCT04352400 {published data only}
-
- NCT04352400.Efficacy of nafamostat in COVID-19 patients (RACONA Study) (RACONA). clinicaltrials.gov/ct2/show/NCT04352400 (first posted 20 April 2020).
NCT04366960 {published data only}
-
- NCT04366960.Comparison of two doses of enoxaparin for thromboprophylaxis in hospitalized COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04366960 (first received 29 April 2020).
NCT04367831 {published data only}
-
- NCT04367831.Intermediate or prophylactic-dose anticoagulation for venous or arterial thromboembolism in severe COVID-19: a cluster based randomized selection trial (IMPROVE-COVID). clinicaltrials.gov/ct2/show/NCT04367831 (first received 27 April 2020).
NCT04373707 {published data only}
-
- NCT04373707.Effectiveness of weight-adjusted prophylactic low molecular weight heparin doses compared with lower fixed prophylactic doses to prevent venous thromboembolism in COVID-2019. The multicenter randomized controlled open-label trial COVI-DOSE. clinicaltrials.gov/ct2/show/record/NCT04373707 (first received 4 May 2020).
NCT04377997 {published data only}
-
- NCT04377997.A randomized, open-label trial of therapeutic anticoagulation in COVID-19 patients with an elevated D-dimer. clinicaltrials.gov/ct2/show/NCT04377997 (first received 7 May 2020).
NCT04397510 {published data only}
-
- NCT04397510.Nebulized heparin vs. placebo for the treatment of COVID-19 induced lung injury. clinicaltrials.gov/ct2/show/NCT04397510 (first received 21 May 2020).
NCT04406389 {published data only}
-
- NCT04406389.Anticoagulation in critically ill patients with COVID-19 (The IMPACT Trial). www.clinicaltrials.gov/ct2/show/NCT04406389 (first received 26 May 2020).
NCT04409834 {published data only}
-
- NCT04409834.Prevention of arteriovenous thrombotic events in critically-ill COVID-19 patients trial (COVID-PACT). clinicaltrials.gov/ct2/show/NCT04409834 (first received 28 May 2020).
NCT04416048 {published data only}
-
- EUCTR2020-002282-33-DE.Effect of anticoagulation therapy on clinical outcomes in COVID-19 COVID-PREVENT. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=NCT04416048 (first received 30 November 2020).
-
- NCT04416048.Effect of anticoagulation therapy on clinical outcomes in moderate to severe coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04416048 (first received 4 June 2020).
NCT04420299 {published data only}
-
- NCT04420299.Clinical trial on the efficacy and safety of bemiparin in patients hospitalized because of COVID-19. clinicaltrials.gov/ct2/show/NCT04420299 (first received 5 June 2020).
NCT04444700 {published data only}
-
- NCT04444700.A pragmatic randomized controlled trial of therapeutic anticoagulation versus standard care as a rapid response to (SARS-CoV-2) COVID-19 pandemic (RAPID-BRAZIL). clinicaltrials.gov/ct2/show/NCT04444700 (first received 22 June 2020).
NCT04485429 {published data only}
-
- NCT04485429.Efficacy assessment of methylprednisolone and heparin in patients with COVID-19 Pneumonia. clinicaltrials.gov/ct2/show/NCT04485429 (first received 20 July 2020).
NCT04508439 {published data only}
-
- NCT04508439.Effect of the use of anticoagulant therapy during hospitalization and discharge in patients with COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04508439 (first received 5 August 2020).
NCT04511923 {published data only}
-
- NCT04511923.Nebulised heparin to reduce COVID-19 induced acute lung injury (CHARTER-Irl). clinicaltrials.gov/ct2/show/NCT04511923 (first received 10 August 2020).
NCT04512079 {published data only}
-
- NCT04512079.Freedom COVID-19 anticoagulation strategy (FREEDOM COVID). clinicaltrials.gov/ct2/show/NCT04512079 (first received 11 August 2020).
NCT04530578 {published data only}
-
- NCT04530578.Nebulized heparin in severe scute respiratory syndrome COVID-19 (NEBUHEPA). clinicaltrials.gov/ct2/show/NCT04530578 (first received 17 August 2020).
NCT04542408 {published data only}
-
- NCT04542408.Hamburg edoxaban for anticoagulation in COVID-19 study. clinicaltrials.gov/ct2/show/NCT04542408 (first received 6 September 2020).
NCT04545541 {published data only}
-
- NCT04545541.Nebulised heparin in patients with severe COVID-19 (CHARTER-MT). clinicaltrials.gov/ct2/show/NCT04545541 (first received 5 September 2020).
NCT04584580 {published data only}
-
- NCT04584580.D-dimer adjusted versus therapeutic dose low-molecular-weight heparin in patients with COVID-19 pneumonia. clinicaltrials.gov/ct2/show/NCT04584580 (first received 6 October 2020).
NCT04600141 {published data only}
-
- NCT04600141.Clinical efficacy of heparin and tocilizumab in patients with severe COVID-19 infection: a randomized clinical trial. clinicaltrials.gov/ct2/show/NCT04600141 (first received 22 October 2020).
NCT04604327 {published data only}
-
- NCT04604327.Comparison of two different doses of bemiparin in COVID-19 (BEMICOP). clinicaltrials.gov/ct2/show/NCT04604327 (first received 23 October 2020).
NCT04623177 {published data only}
-
- NCT04623177.Thromboprophylaxis for patients in ICU with COVID-19. clinicaltrials.gov/ct2/show/NCT04623177 (first received 02 November 2020).
NCT04640181 {published data only}
-
- NCT04640181.Factor Xa inhibitor versus standard of care heparin in hospitalized patients with COVID-19 (XACT). clinicaltrials.gov/ct2/show/NCT04640181 (first received 19 November 2020).
NCT04646655 {published data only}
-
- NCT04646655.Enoxaparin at prophylactic or therapeutic doses in COVID-19 (EMOS-COVID). clinicaltrials.gov/ct2/show/NCT04646655?term=NCT04646655&draw=2&... (first received 30 November 2020).
NCT04655586 {published data only}
-
- NCT04655586.Assessing safety, hospitalization and efficacy of rNAPc2 in COVID-19 (ASPEN). clinicaltrials.gov/ct2/show/NCT04655586?term=Assessing+Safety%2C+Hospita... (first received 7 December 2020).
NCT04723563 {published data only}
-
- NCT04723563.Nebulized heparin for the treatment of COVID-19 (INHALE-HEP). clinicaltrials.gov/ct2/show/NCT04723563 (first received 22 January 2021).
NCT04730856 {published data only}
-
- NCT04730856.Standard vs high prophylactic doses or anticoagulation in patients with high risk of thrombosis admitted with COVID-19 pneumonia (PROTHROMCOVID). clinicaltrials.gov/ct2/show/NCT04730856 (first received 14 January 2021).
NCT04743011 {published data only}
-
- NCT04743011.Enriched heparin anti COVID-19 trial (EnHanCed). clinicaltrials.gov/ct2/show/NCT04743011 (first received 3 February 2021).
NCT04745442 {published data only}
-
- NCT04745442.Pilot study of antithrombin as prophylaxis of acute respiratory distress syndrome in patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04745442 (first received 8 February 2021).
PACTR202007606032743 {published data only}
-
- PACTR202007606032743.Nebulized heparin in patients with mainly moderate coronavirus disease 2019: randomized controlled trial. COVID-19. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=PACTR202007606032743 (first received 15 June 2020).
RBR‐7y8j2bs {published data only}
-
- RBR-7y8j2bs.Inhalational high molecular weight heparin for the treatment of SARS-Cov-2. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=RBR-7y8j2bs (first received 23 February 2021).
Sholzberg 2021a {published data only}
-
- NCT04362085.Coagulopathy of COVID-19: a pragmatic randomized controlled trial of therapeutic anticoagulation versus standard care as a rapid response to the COVID-19 pandemic (RAPID COVID COAG). clinicaltrials.gov/ct2/show/NCT04362085 (first received 24 April 2020).
-
- Sholzberg M, Tang GH, Negri E, Rahhal H, Kreuziger LB, Pompilio CE, et al.Coagulopathy of hospitalised COVID-19: a pragmatic randomised controlled trial of therapeutic anticoagulation versus standard care as a rapid response to the COVID-19 pandemic (RAPID COVID COAG - RAPID Trial): a structured summary of a study protocol for a randomised controlled trial. Trials 2021;22(1):202. [DOI: 10.1186/s13063-021-05076-0] - DOI - PMC - PubMed
Vanassche 2020 {published data only}
-
- EUCTR2020-001739-28-BE.A randomized, open-label, adaptive, proof-of-concept clinical trial of modulation of host thromboinflammatory response in patients with COVID-19. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=EUCTR2020-001739-28-BE (first received 10 April 2020).
-
- Vanassche T, Engelen MM, Van Thillo Q, Wauters J, Gunst J, Wouters C, et al.A randomized, open-label, adaptive, proof-of-concept clinical trial of modulation of host thromboinflammatory response in patients with COVID-19: the DAWn-Antico study. Trials 2020;21:1005. [DOI: 10.1186/s13063-020-04878-y] - DOI - PMC - PubMed
-
- Vanassche T, Engelen MM, Van Thillo Q, Wauters J, Gunst J, Wouters C, et al.Correction to: A randomized, open-label, adaptive, proof-of-concept clinical trial of modulation of host thromboinflammatory response in patients with COVID-19: the DAWn-Antico study. Trials 2020;21:1033. [DOI: 10.1186/s13063-020-04991-y] - DOI - PMC - PubMed
Van Haren 2020 {published data only}
-
- NCT04635241.Inhaled heparin for hospitalised COVID-19 patients (INHALE-HEP). clinicaltrials.gov/ct2/show/NCT04635241 (first received 19 November 2020).
-
- Van Haren FMP, Richardson A, Yoon H-J, Artigas A, Laffey JG, Dixon B, et al.Inhaled nebulised unfractionated heparin for the treatment of hospitalised patients with COVID-19 (INHALE-HEP): protocol and statistical analysis plan for an investigator-initiated international metatrial of randomised studies. British Journal of Clinical Pharmacology 2020;88(8):3075-91. [DOI: 10.1111/bcp.14714] - DOI - PubMed
Wilkinson 2020 {published data only}
-
- EudraCT2020-001736-95.ACCORD 2: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID 19 in hospitalised patients. clinicaltrialsregister.eu/ctr-search/trial/2020-001736-95/GB (first received 23 April 2020). - PMC - PubMed
-
- ISRCTN57085639.A platform to investigate the safety and effectiveness of several new medicines for the treatment of COVID-19 in hospitalised patients. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN57085639 (first received 24 September 2020).
-
- Wilkinson T, Dixon R, Page C, Carroll M, Griffiths G, Ho L-P, et al.ACCORD: a multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID-19 in hospitalised patients: a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):691. [DOI: 10.1186/s13063-020-04584-9] - DOI - PMC - PubMed
Additional references
Abdel‐Maboud 2021
Ackermann 2020
Alquwaizani 2013
Amaral 2020
AVF 2020
-
- American Venous Forum.Considerations in prophylaxis and treatment of VTE in COVID-19 patients. www.veinforum.org/wp-content/uploads/2020/04/COVID-19-White-Paper-04-17-... (accessed 3 July 2020).
Becker 2020
Biagioni 2020
-
- Biagioni RB, Lopes RD, Agati LB, Sacilotto R, Wolosker N, Sobreira ML, et al.Rationale and design for the study apixaban versus clopidogrel on a background of aspirin in patients undergoing infrapopliteal angioplasty for critical limb ischemia: AGRIPPA trial. American Heart Journal 2020;227:100-6. [DOI: 10.1016/j.ahj.2020.06.010] - DOI - PubMed
Bikdeli 2020
-
- Bikdeli B, Madhavan M, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al.COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state of the art review. Journal of American College of Cardiology 2020;75(23):2950-73. [DOI: 10.1016/j.jacc.2020.04.031] - DOI - PMC - PubMed
Bilaloglu 2020
Clezar 2020
COMET 2020
-
- Core outcome set developers’ response to COVID-19. comet-initiative.org/Studies/Details/1538 (accessed 04 August 2020).
Covidence [Computer program]
-
- Covidence.Version accessed 1 August 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
COVIDSurg 2021
Cuker 2021
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG, editor(s).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Dias 2021
Dolhnikoff 2020
Fox 2020
Giannis 2020
GRADEpro GDT [Computer program]
-
- GRADEpro GDT.Version accessed 1 August 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Hasan 2020
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s).Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2020a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors).Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Higgins 2020b
-
- Higgins JP, Eldridge S, Li T, editor(s).Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Kamel 2021
Klok 2020a
Klok 2020b
Lai 2020
-
- Lai CC, Wang CY, Wang YH, Hsueh SC, Ko WC, Hsueh PR.Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. International Journal of Antimicrobial Agents 2020;55(4):105946. [PMID: ] - PMC - PubMed
Lazaridis 2021
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Li 2020
Liu 2019
-
- Liu Y, Mu S, Li X, Liang Y, Wang L, Ma X.Unfractionated heparin alleviates sepsis-induced acute lung injury by protecting tight junctions. Journal of Surgical Research 2019;238:175-85. [PMID: ] - PubMed
Long 2020
Marini 2020
Matli 2021
McBane 2020
McGuinness 2020
Moonla 2021
NHS 2020
-
- NHS England and NHS Improvement.Clinical guide for the management of anticoagulant services during the coronavirus pandemic. www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/C0077... (accessed 3 July 2020).
Obe 2020
-
- Obe BH, Retter A, McClintock C.Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. thrombosisuk.org/downloads/T&H%20and%20COVID.pdf (accessed 3 July 2020).
Oxley 2020
Page 2021
Panigada 2020
-
- Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al.Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. Journal of Thrombosis and Haemostasis 2020;18(7):1738-42. [PMID: ] - PMC - PubMed
Parisi 2021
-
- Parisi R, Costanzo S, Di Castelnuovo A, Gaetano G, Donati MB, Iacoviello L.Different anticoagulant regimens, mortality, and bleeding in hospitalized patients with COVID-19: a systematic review and an updated meta-analysis. Seminars in Thrombosis and Hemostasis 2021;47(4):372-91. [PMID: ] - PubMed
Patell 2021
Perepu 2021
-
- Perepu US, Chambers I, Wahab A, Eyck PT, Wu C, Dayal S, et al.Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. Journal of Thrombosis and Haemostasis 2021;19(9):2225-34. [DOI: 10.1111/jth.15450] - DOI - PMC - PubMed
Ramacciotti 2020
Reeves 2021
-
- Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA.Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Robvis [Computer program]
-
- Robvis (visualization tool).Version accessed 20 August 2020. Bristol, UK: Luke McGuinness. Available at mcguinlu.shinyapps.io/robvis/.
Schulman 2010
-
- Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W.Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. Journal of Thrombosis and Haemostasis : JTH 2010;8(1):202-4. [PMID: ] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s).Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html.
Schünemann 2021
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Sholzberg 2021b
-
- Sholzberg M, Tang GH, Rahhal H, AlHamzah M, Kreuziger LB, Ní Áinle F, et al.Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021;375:n2400. [DOI: 10.1136/bmj.n2400] - DOI - PMC - PubMed
Sobreira 2020
Spyropoulos 2021
-
- Spyropoulos AC, Goldin M, Giannis D, Diab W, Wang J, Khanijo S, et al.Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Internal Medicine 2021 October 7 [Epub ahead of print]. [DOI: 10.1001/jamainternmed.2021.6203] - DOI - PMC - PubMed
Sterne 2016
Talasaz 2021
-
- Talasaz AH, Sadeghipour P, Kakavand H, Aghakouchakzadeh M, Kordzadeh-Kermani E, Van Tassell BW, et al.Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC state-of-the-art review. Journal of the American College of Cardiology 2021;77(15):1903-21. [PMID: ] - PMC - PubMed
Wan 2014
Ware 1992
-
- Ware JE Jr, Sherbourne CD.The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] - PubMed
Young 2008
-
- Young E.The anti-inflammatory effects of heparin and related compounds. Thrombosis Research 2008;122(6):743-52. [PMID: ] - PubMed
References to other published versions of this review
Flumignan 2020a
-
- Flumignan RL, Tinôco JD, Pascoal PI, Areias LL, Cossi MS, Fernandes MI, et al.Prophylactic anticoagulants for patients hospitalised with COVID-19 (Protocol). available from doi.org/10.17605/OSF.IO/8PRXW (registered 7 August 2020).
Flumignan 2020b
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

