Yunpi Heluo decoction reduces ectopic deposition of lipids by regulating the SIRT1-FoxO1 autophagy pathway in diabetic rats
- PMID: 35244516
- PMCID: PMC8916783
- DOI: 10.1080/13880209.2022.2042567
Yunpi Heluo decoction reduces ectopic deposition of lipids by regulating the SIRT1-FoxO1 autophagy pathway in diabetic rats
Abstract
Context: Yunpi Heluo (YPHL) decoction is a Chinese herbal formula with particular advantages for treating type 2 diabetes. Yet, its exact mechanism of action is not fully understood.
Objective: To examine the therapeutic effect of YPHL on ectopic lipid deposition (EDL) in Zucker diabetic fatty (ZDF) rats and the underlying mechanism.
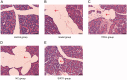
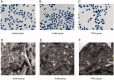
Materials and methods: The ZDF Rats were randomized into five groups, including model, YPHL (200 mg/kg/d for 10 weeks), SIRT1-overexpression (injected with HBAAV2/9-r-SIRT1-3'-flag-GFP), NC (injected with HBAAV2/9-CMV-GFP as blank control) and control group. Pancreatic β-cells obtained from high-lipid-high-glucose fed rats were treated with YPHL (10 mg/mL) for 48 h. Lipid deposition and autophagosomes were analyzed by transmission electron microscopy. Intracellular H2O2 and ROS concentrations were measured by flow cytometry. SIRT1, FOXO1, LC3 and P62 mRNA and protein levels were analyzed using qRT-PCR and Western blots.
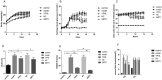
Results: Compared with the model group, blood glucose levels in YPHL and si-SIRT1 groups were reduced by 19.3% and 27.9%, respectively. In high-lipid-high-glucose cells treated with YPHL, lipid droplets were reduced and decrease in apoptosis rate (38.6%), H2O2 (31.2%) and ROS (44.5%) levels were observed. After YPHL intervention or SIRT1 overexpression, LC3 and p62 expression increased. Protein expression of SIRT1 and LC3 in model, si-SIRT1, si-NC and si-SIRT1 + YPHL groups was lower than those in control group, while FoxO1 expression was increased. All of these protein level alterations were reversed in the si-NC + YPHL group.
Discussion and conclusions: YPHL reduced EDL by regulating the SIRT1-FoxO1 autophagy pathway in diabetic rats, which could lead to future perspectives for the treatment of diabetes.
Keywords: Insulin resistance; pancreas; type 2 diabetes.
Conflict of interest statement
The authors report no conflict of interest.
Figures




Similar articles
-
Yunpi Heluo decoction attenuates insulin resistance by regulating SIRT1-FoxO1 autophagy pathway in skeletal muscle of Zucker diabetic fatty rats.J Ethnopharmacol. 2021 Apr 24;270:113828. doi: 10.1016/j.jep.2021.113828. Epub 2021 Jan 18. J Ethnopharmacol. 2021. PMID: 33476712
-
Yunpi Heluo decoction attenuates insulin resistance by regulating liver miR-29a-3p in Zucker diabetic fatty rats.J Ethnopharmacol. 2019 Oct 28;243:111966. doi: 10.1016/j.jep.2019.111966. Epub 2019 May 23. J Ethnopharmacol. 2019. PMID: 31128151
-
Effect and mechanism of Angelic Shaoyaosan mediated AMPK/SIRT1 positive feedback loop to promote autophagy and regulate the systemic inflammatory response in acute pancreatitis.Cell Mol Biol (Noisy-le-grand). 2021 Aug 31;67(2):101-108. doi: 10.14715/cmb/2021.67.2.15. Cell Mol Biol (Noisy-le-grand). 2021. PMID: 34817332
-
Yishen capsule promotes podocyte autophagy through regulating SIRT1/NF-κB signaling pathway to improve diabetic nephropathy.Ren Fail. 2021 Dec;43(1):128-140. doi: 10.1080/0886022X.2020.1869043. Ren Fail. 2021. PMID: 33427556 Free PMC article.
-
Sirtuin 1 as the mechanism of action of agents used in the diabetes mellitus pharmacotherapy.Eur J Pharmacol. 2021 Sep 15;907:174289. doi: 10.1016/j.ejphar.2021.174289. Epub 2021 Jun 30. Eur J Pharmacol. 2021. PMID: 34214583 Review.
Cited by
-
A novel strategy for the protective effect of ginsenoside Rg1 against ovarian reserve decline by the PINK1 pathway.Pharm Biol. 2025 Dec;63(1):68-81. doi: 10.1080/13880209.2025.2453699. Epub 2025 Jan 25. Pharm Biol. 2025. PMID: 39862058 Free PMC article.
-
Establishment of a Steatosis Model in LMH Cells, Chicken Embryo Hepatocytes, and Liver Tissues Based on a Mixture of Sodium Oleate and Palmitic Acid.Animals (Basel). 2024 Jul 26;14(15):2173. doi: 10.3390/ani14152173. Animals (Basel). 2024. PMID: 39123699 Free PMC article.
-
Yiqi Huazhuo decoction increases insulin secretion in type 2 diabetic rats by regulating the pancreatic GPR40-IP3R-1 signaling pathway.Front Pharmacol. 2023 Mar 14;14:1136778. doi: 10.3389/fphar.2023.1136778. eCollection 2023. Front Pharmacol. 2023. PMID: 36998612 Free PMC article.
-
LITAF inhibits colorectal cancer stemness and metastatic behavior by regulating FOXO1-mediated SIRT1 expression.Clin Exp Metastasis. 2023 Aug;40(4):309-320. doi: 10.1007/s10585-023-10213-x. Epub 2023 Jun 2. Clin Exp Metastasis. 2023. PMID: 37266842
-
Component-Effect Relationship between HPLC Fingerprints and Lipid-Lowering Activity of Buyang Huanwu Decoction.Int J Anal Chem. 2022 Sep 26;2022:9195335. doi: 10.1155/2022/9195335. eCollection 2022. Int J Anal Chem. 2022. PMID: 36199444 Free PMC article.
References
-
- Chai K, Zhang X, Dai M.. 2013. Influence of Yunpi Heluo granule on proliferation of rat Schwann cells cultured in high glucose and expression of NGF mRNA. Chinese Arch Trad Chinese Med (Chinese). 60:970–973.
-
- Chan M. 2017. China's burgeoning epidemic of diabetes-associated mortality. JAMA. 317(1):264–266. - PubMed
-
- Etgen GJ, Oldham BA.. 2000. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism. 49(5):684–688. - PubMed
-
- Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, Buckingham RE.. 2001. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 50(5):1021–1029. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
