Maturation of beta cells: lessons from in vivo and in vitro models
- PMID: 35244743
- PMCID: PMC9076740
- DOI: 10.1007/s00125-022-05672-y
Maturation of beta cells: lessons from in vivo and in vitro models
Abstract
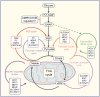
The ability to maintain normoglycaemia, through glucose-sensitive insulin release, is a key aspect of postnatal beta cell function. However, terminally differentiated beta cell identity does not necessarily imply functional maturity. Beta cell maturation is therefore a continuation of beta cell development, albeit a process that occurs postnatally in mammals. Although many important features have been identified in the study of beta cell maturation, as of yet no unified mechanistic model of beta cell functional maturity exists. Here, we review recent findings about the underlying mechanisms of beta cell functional maturation. These findings include systemic hormonal and nutritional triggers that operate through energy-sensing machinery shifts within beta cells, resulting in primed metabolic states that allow for appropriate glucose trafficking and, ultimately, insulin release. We also draw attention to the expansive synergistic nature of these pathways and emphasise that beta cell maturation is dependent on overlapping regulatory and metabolic networks.
Keywords: AMPK; Beta cells; Circadian; Differentiation; Islets; Maturation; Metabolism; Review; Stem cells; mTOR.
© 2022. The Author(s).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

