Canine osteosarcoma cells exhibit basal accumulation of multiple chaperone proteins and are sensitive to small molecule inhibitors of GRP78 and heat shock protein function
- PMID: 35244890
- PMCID: PMC9106791
- DOI: 10.1007/s12192-022-01263-3
Canine osteosarcoma cells exhibit basal accumulation of multiple chaperone proteins and are sensitive to small molecule inhibitors of GRP78 and heat shock protein function
Abstract
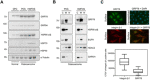
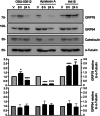
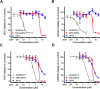
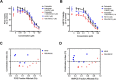
Osteosarcoma is the most common type of bone cancer in dogs and humans, with significant numbers of patients experiencing treatment failure and disease progression. In our search for new approaches to treat osteosarcoma, we previously detected multiple chaperone proteins in the surface-exposed proteome of canine osteosarcoma cells. In the present study, we characterized expression of representative chaperones and find evidence for stress adaptation in canine osteosarcoma cells relative to osteogenic progenitors from normal bone. We compared the cytotoxic potential of direct (HA15) and putative (OSU-03012) inhibitors of Grp78 function and found canine POS and HMPOS osteosarcoma cells to be more sensitive to both compounds than normal cells. HA15 and OSU-03012 increased the thermal stability of Grp78 in intact POS cells at low micromolar concentrations, but each induced distinct patterns in Grp78 expression without significant change in Grp94. Both inhibitors were as effective alone as carboplatin and showed little evidence of synergy in combination treatment. However, HMPOS cells with acquired resistance to carboplatin were sensitive to inhibition of Grp78 (by HA15; OSU-03012), Hsp70 (by VER-155008), and Hsp90 (by 17-AAG) function. These results suggest that multiple nodes within the osteosarcoma chaperome may be relevant chemotherapeutic targets against platinum resistance.
Keywords: AR-12; BiP; Chaperome; HA15; HSP5A; OSU-03012; Proteostasis.
© 2022. The Author(s) under exclusive licence to [Cell Stress Society International.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Targeting HSP70 and GRP78 in canine osteosarcoma cells in combination with doxorubicin chemotherapy.Cell Stress Chaperones. 2016 Nov;21(6):1065-1076. doi: 10.1007/s12192-016-0730-4. Epub 2016 Sep 8. Cell Stress Chaperones. 2016. PMID: 27631331 Free PMC article.
-
OSU-03012 stimulates PKR-like endoplasmic reticulum-dependent increases in 70-kDa heat shock protein expression, attenuating its lethal actions in transformed cells.Mol Pharmacol. 2008 Apr;73(4):1168-84. doi: 10.1124/mol.107.042697. Epub 2008 Jan 8. Mol Pharmacol. 2008. PMID: 18182481 Free PMC article.
-
Inhibition of copper chaperones sensitizes human and canine osteosarcoma cells to carboplatin chemotherapy.Vet Comp Oncol. 2020 Dec;18(4):559-569. doi: 10.1111/vco.12579. Epub 2020 Mar 20. Vet Comp Oncol. 2020. PMID: 32060984
-
GRP78 in lung cancer.J Transl Med. 2021 Mar 21;19(1):118. doi: 10.1186/s12967-021-02786-6. J Transl Med. 2021. PMID: 33743739 Free PMC article. Review.
-
Molecular Chaperones in Osteosarcoma: Diagnosis and Therapeutic Issues.Cells. 2021 Mar 30;10(4):754. doi: 10.3390/cells10040754. Cells. 2021. PMID: 33808130 Free PMC article. Review.
Cited by
-
Genomic loss of the HSP70cA gene in the vertebrate lineage.Cell Stress Chaperones. 2023 Nov;28(6):1053-1067. doi: 10.1007/s12192-023-01370-9. Epub 2023 Aug 17. Cell Stress Chaperones. 2023. PMID: 37587350 Free PMC article.
-
Endoplasmic reticulum stress and therapeutic strategies in metabolic, neurodegenerative diseases and cancer.Mol Med. 2024 Mar 20;30(1):40. doi: 10.1186/s10020-024-00808-9. Mol Med. 2024. PMID: 38509524 Free PMC article. Review.
-
Diastereomers of Coibamide A Show Altered Sec61 Client Selectivity and Ligand-Dependent Activity against Patient-Derived Glioma Stem-like Cells.ACS Pharmacol Transl Sci. 2024 May 14;7(6):1823-1838. doi: 10.1021/acsptsci.4c00049. eCollection 2024 Jun 14. ACS Pharmacol Transl Sci. 2024. PMID: 38898945 Free PMC article.
-
Chaperone-Mediated Responses and Mitochondrial-Endoplasmic Reticulum Coupling: Emerging Insight into Alzheimer's Disease.Cells. 2025 Jul 31;14(15):1179. doi: 10.3390/cells14151179. Cells. 2025. PMID: 40801612 Free PMC article. Review.
-
Synthetic Small Molecule Modulators of Hsp70 and Hsp40 Chaperones as Promising Anticancer Agents.Int J Mol Sci. 2023 Feb 17;24(4):4083. doi: 10.3390/ijms24044083. Int J Mol Sci. 2023. PMID: 36835501 Free PMC article. Review.
References
-
- Bhattacharjee R, Devi A, Mishra S. Molecular docking and molecular dynamics studies reveal structural basis of inhibition and selectivity of inhibitors EGCG and OSU-03012 toward glucose regulated protein-78 (GRP78) overexpressed in glioblastoma. J Mol Model. 2015;21(10):272. doi: 10.1007/s00894-015-2801-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

